Deubiquitylation and stabilization of p21 by USP11 is critical for cell-cycle progression and DNA damage responses
- PMID: 29666278
- PMCID: PMC5939064
- DOI: 10.1073/pnas.1714938115
Deubiquitylation and stabilization of p21 by USP11 is critical for cell-cycle progression and DNA damage responses
Abstract
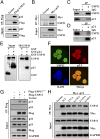
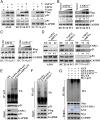
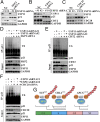
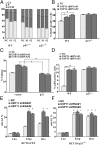
p21WAF1/CIP1 is a broad-acting cyclin-dependent kinase inhibitor. Its stability is essential for proper cell-cycle progression and cell fate decision. Ubiquitylation by the multiple E3 ubiquitin ligase complexes is the major regulatory mechanism of p21, which induces p21 degradation. However, it is unclear whether ubiquitylated p21 can be recycled. In this study, we report USP11 as a deubiquitylase of p21. In the nucleus, USP11 binds to p21, catalyzes the removal of polyubiquitin chains conjugated onto p21, and stabilizes p21 protein. As a result, USP11 reverses p21 polyubiquitylation and degradation mediated by SCFSKP2, CRL4CDT2, and APC/CCDC20 in a cell-cycle-independent manner. Loss of USP11 causes the destabilization of p21 and induces the G1/S transition in unperturbed cells. Furthermore, p21 accumulation mediated by DNA damage is completely abolished in cells depleted of USP11, which results in abrogation of the G2 checkpoint and induction of apoptosis. Functionally, USP11-mediated stabilization of p21 inhibits cell proliferation and tumorigenesis in vivo. These findings reveal an important mechanism by which p21 can be stabilized by direct deubiquitylation, and they pinpoint a crucial role of the USP11-p21 axis in regulating cell-cycle progression and DNA damage responses.
Keywords: USP11; cell cycle; deubiquitylation; p21; ubiquitin.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

