Biofilms Comprise a Component of the Annual Cycle of Vibrio cholerae in the Bay of Bengal Estuary
- PMID: 29666284
- PMCID: PMC5904415
- DOI: 10.1128/mBio.00483-18
Biofilms Comprise a Component of the Annual Cycle of Vibrio cholerae in the Bay of Bengal Estuary
Abstract
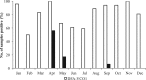
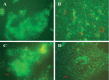
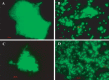
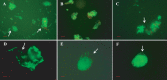
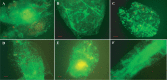
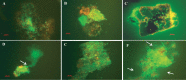
Vibrio cholerae, an estuarine bacterium, is the causative agent of cholera, a severe diarrheal disease that demonstrates seasonal incidence in Bangladesh. In an extensive study of V. cholerae occurrence in a natural aquatic environment, water and plankton samples were collected biweekly between December 2005 and November 2006 from Mathbaria, an estuarine village of Bangladesh near the mangrove forests of the Sundarbans. Toxigenic V. cholerae exhibited two seasonal growth peaks, one in spring (March to May) and another in autumn (September to November), corresponding to the two annual seasonal outbreaks of cholera in this region. The total numbers of bacteria determined by heterotrophic plate count (HPC), representing culturable bacteria, accounted for 1% to 2.7% of the total numbers obtained using acridine orange direct counting (AODC). The highest bacterial culture counts, including toxigenic V. cholerae, were recorded in the spring. The direct fluorescent antibody (DFA) assay was used to detect V. cholerae O1 cells throughout the year, as free-living cells, within clusters, or in association with plankton. V. cholerae O1 varied significantly in morphology, appearing as distinctly rod-shaped cells in the spring months, while small coccoid cells within thick clusters of biofilm were observed during interepidemic periods of the year, notably during the winter months. Toxigenic V. cholerae O1 was culturable in natural water during the spring when the temperature rose sharply. The results of this study confirmed biofilms to be a means of persistence for bacteria and an integral component of the annual life cycle of toxigenic V. cholerae in the estuarine environment of Bangladesh.IMPORTANCEVibrio cholerae, the causative agent of cholera, is autochthonous in the estuarine aquatic environment. This study describes morphological changes in naturally occurring V. cholerae O1 in the estuarine environment of Mathbaria, where the bacterium is culturable when the water temperature rises and is observable predominantly as distinct rods and dividing cells. In the spring and fall, these morphological changes coincide with the two seasonal peaks of endemic cholera in Bangladesh. V. cholerae O1 cells are predominantly coccoid within biofilms but are rod shaped as free-living cells and when attached to plankton or to particulate matter in interepidemic periods of the year. It is concluded that biofilms represent a stage of the annual life cycle of V. cholerae O1, the causative agent of cholera in Bangladesh.
Keywords: Vibrio cholerae; biofilms; cholera.
Copyright © 2018 Sultana et al.
Figures


References
-
- Morita RY. 1985. Starvation and miniaturization of heterotrophs with special emphasis on the maintenance of the starved viable state, p 111–130. In Fletcher M, Floodgate G (ed), Bacteria in the natural environments: the effect of nutrient conditions. Academic Press, New York, NY.
-
- Oliver JD. 2000. The viable but nonculturable state and cellular resuscitation, p 723–730. In Bell CR, Brylinsky M, Johnson-Green P (ed), Microbial biosystems: new frontiers. Proceedings of the 8th International Symposium on Microbial Ecology. Atlantic Canada Society, Halifax, Canada.
-
- Oliver JD. 2005. The viable but nonculturable state in bacteria. J Microbiol 43(Spec No):93−100. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

