Delayed Autoimmune Toxicity Occurring Several Months After Cessation of Anti-PD-1 Therapy
- PMID: 29666298
- PMCID: PMC6058335
- DOI: 10.1634/theoncologist.2017-0531
Delayed Autoimmune Toxicity Occurring Several Months After Cessation of Anti-PD-1 Therapy
Abstract
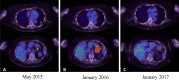
Treatment with anti-programmed cell death protein 1 (PD-1) antibodies has demonstrated clinical efficacy in a whole range of malignancies including advanced melanoma, renal cell cancer, bladder cancer, and non-small cell lung cancer. Immune-related adverse events are a unique side effect of checkpoint regulator therapy including anti-PD-1 antibodies. Treatment-related autoimmunity can occur in any organ system, with the median onset usually within 5-15 weeks from the commencement of therapy, depending on the organ system involved. This study describes for the first time a case of delayed autoimmunity occurring 8 months after discontinuing treatment with the anti-PD-1 antibody nivolumab in a patient with metastatic melanoma. The case highlights the need for ongoing surveillance of patients treated with immune checkpoint inhibitors even after cessation of therapy, especially as patients increasingly stop treatment after achieving durable responses.
© AlphaMed Press 2018.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

References
-
- Callahan MK, Postow MA, Wolchok JD. Targeting T cell co‐receptors for cancer therapy. Immunity 2016;44:1069–1078. - PubMed
-
- Weber JS, Hodi FS, Wolchok JD et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 2016;35:785–792. - PubMed
-
- Larkin J, Chiarion‐Sileni V, Gonzalez R et al., eds. Overall survival results from a phase III trial of nivolumab combined with ipilimumab in treatment‐naïve patients with advanced melanoma (CheckMate 067). Proceedings from the American Association for Cancer Research Annual Meeting; April 1–5, 2017; Washington, DC.
-
- Schachter J, Ribas A, Long GV et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open‐label phase 3 study (KEYNOTE‐006). Lancet 2017;390:1853–1862. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

