Improved calcium sensor GCaMP-X overcomes the calcium channel perturbations induced by the calmodulin in GCaMP
- PMID: 29666364
- PMCID: PMC5904127
- DOI: 10.1038/s41467-018-03719-6
Improved calcium sensor GCaMP-X overcomes the calcium channel perturbations induced by the calmodulin in GCaMP
Abstract
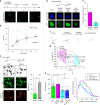
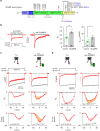
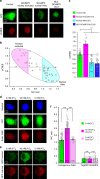
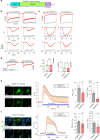
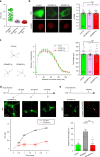
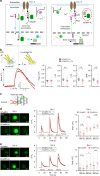
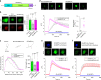
GCaMP, one popular type of genetically-encoded Ca2+ indicator, has been associated with various side-effects. Here we unveil the intrinsic problem prevailing over different versions and applications, showing that GCaMP containing CaM (calmodulin) interferes with both gating and signaling of L-type calcium channels (CaV1). GCaMP acts as an impaired apoCaM and Ca2+/CaM, both critical to CaV1, which disrupts Ca2+ dynamics and gene expression. We then design and implement GCaMP-X, by incorporating an extra apoCaM-binding motif, effectively protecting CaV1-dependent excitation-transcription coupling from perturbations. GCaMP-X resolves the problems of detrimental nuclear accumulation, acute and chronic Ca2+ dysregulation, and aberrant transcription signaling and cell morphogenesis, while still demonstrating excellent Ca2+-sensing characteristics partly inherited from GCaMP. In summary, CaM/CaV1 gating and signaling mechanisms are elucidated for GCaMP side-effects, while allowing the development of GCaMP-X to appropriately monitor cytosolic, submembrane or nuclear Ca2+, which is also expected to guide the future design of CaM-based molecular tools.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

