Attenuated DNA damage responses and increased apoptosis characterize human hematopoietic stem cells exposed to irradiation
- PMID: 29666389
- PMCID: PMC5904119
- DOI: 10.1038/s41598-018-24440-w
Attenuated DNA damage responses and increased apoptosis characterize human hematopoietic stem cells exposed to irradiation
Abstract
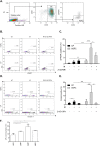
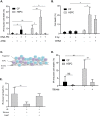
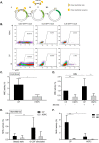
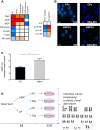
Failure to precisely repair DNA damage in self-renewing Hematopoietic Stem and early Progenitor Cells (HSPCs) can disrupt normal hematopoiesis and promote leukemogenesis. Although HSPCs are widely considered a target of ionizing radiation (IR)-induced hematopoietic injury, definitive data regarding cell death, DNA repair, and genomic stability in these rare quiescent cells are scarce. We found that irradiated HSPCs, but not lineage-committed progenitors (CPs), undergo rapid ATM-dependent apoptosis, which is suppressed upon interaction with bone-marrow stroma cells. Using DNA repair reporters to quantify mutagenic Non-Homologous End Joining (NHEJ) processes, we found that HSPCs exhibit reduced NHEJ activities in comparison with CPs. HSPC-stroma interactions did not affect the NHEJ capacity of HSPCs, emphasizing its cell autonomous regulation. We noted diminished expression of multiple double strand break (DSB) repair transcripts along with more persistent 53BP1 foci in irradiated HSPCs in comparison with CPs, which can account for low NHEJ activity and its distinct control in HSPCs. Finally, we documented clonal chromosomal aberrations in 10% of IR-surviving HSPCs. Taken together, our results revealed potential mechanisms contributing to the inherent susceptibility of human HSPC to the cytotoxic and mutagenic effects of DNA damage.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

