Intrinsic mechanisms of neuronal axon regeneration
- PMID: 29666508
- PMCID: PMC5987780
- DOI: 10.1038/s41583-018-0001-8
Intrinsic mechanisms of neuronal axon regeneration
Abstract
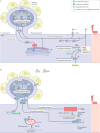
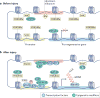
Permanent disabilities following CNS injuries result from the failure of injured axons to regenerate and rebuild functional connections with their original targets. By contrast, injury to peripheral nerves is followed by robust regeneration, which can lead to recovery of sensory and motor functions. This regenerative response requires the induction of widespread transcriptional and epigenetic changes in injured neurons. Considerable progress has been made in recent years in understanding how peripheral axon injury elicits these widespread changes through the coordinated actions of transcription factors, epigenetic modifiers and, to a lesser extent, microRNAs. Although many questions remain about the interplay between these mechanisms, these new findings provide important insights into the pivotal role of coordinated gene expression and chromatin remodelling in the neuronal response to injury.
Figures
References
-
- Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu. Rev. Neurosci. 2011;34:131–152. - PubMed
-
- Di Giovanni S. Molecular targets for axon regeneration: focus on the intrinsic pathways. Expert Opin. Ther. Targets. 2009;13:1387–1398. - PubMed
-
- Tedeschi A, Bradke F. Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr. Opin. Neurobiol. 2017;42:118–127. - PubMed
-
- Kaplan A, Bueno M, Hua L, Fournier AE. Maximizing functional axon repair in the injured central nervous system: lessons from neuronal development. Dev. Dyn. 2017;247:18–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

