Role of Muramyl Dipeptide in Lipopolysaccharide-Mediated Biological Activity and Osteoclast Activity
- PMID: 29666781
- PMCID: PMC5832107
- DOI: 10.1155/2018/8047610
Role of Muramyl Dipeptide in Lipopolysaccharide-Mediated Biological Activity and Osteoclast Activity
Abstract
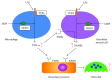
Lipopolysaccharide (LPS) is an endotoxin and bacterial cell wall component that is capable of inducing inflammation and immunological activity. Muramyl dipeptide (MDP), the minimal essential structural unit responsible for the immunological activity of peptidoglycans, is another inflammation-inducing molecule that is ubiquitously expressed by bacteria. Several studies have shown that inflammation-related biological activities were synergistically induced by interactions between LPS and MDP. MDP synergistically enhances production of proinflammatory cytokines that are induced by LPS exposure. Injection of MDP induces lethal shock in mice challenged with LPS. LPS also induces osteoclast formation and pathological bone resorption; MDP enhances LPS induction of both processes. Furthermore, MDP enhances the LPS-induced receptor activator of NF-κB ligand (RANKL) expression and toll-like receptor 4 (TLR4) expression both in vivo and in vitro. Additionally, MDP enhances LPS-induced mitogen-activated protein kinase (MAPK) signaling in stromal cells. Taken together, these findings suggest that MDP plays an important role in LPS-induced biological activities. This review discusses the role of MDP in LPS-mediated biological activities, primarily in relation to osteoclastogenesis.
Figures

References
-
- Khedoe P., de Kleijn S., van Oeveren-Rietdijk A. M., et al. Acute and chronic effects of treatment with mesenchymal stromal cells on LPS-induced pulmonary inflammation, emphysema and atherosclerosis development. PLoS One. 2017;12(9, article e0183741) doi: 10.1371/journal.pone.0183741. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

