Inhibition of alpha7 nicotinic receptors in the ventral hippocampus selectively attenuates reinstatement of morphine-conditioned place preference and associated changes in AMPA receptor binding
- PMID: 29667304
- PMCID: PMC6563460
- DOI: 10.1111/adb.12624
Inhibition of alpha7 nicotinic receptors in the ventral hippocampus selectively attenuates reinstatement of morphine-conditioned place preference and associated changes in AMPA receptor binding
Abstract
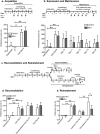
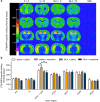
Recurrent relapse is a major problem in treating opiate addiction. Pavlovian conditioning plays a role in recurrent relapse whereby exposure to cues learned during drug intake can precipitate relapse to drug taking. α7 nicotinic acetylcholine receptors (nAChRs) have been implicated in attentional aspects of cognition and mechanisms of learning and memory. In this study we have investigated the role of α7 nAChRs in morphine-conditioned place preference (morphine-CPP). CPP provides a model of associative learning that is pertinent to associative aspects of drug dependence. The α7 nAChR antagonist methyllycaconitine (MLA; 4 mg/kg s.c.) had no effect on the acquisition, maintenance, reconsolidation or extinction of morphine-CPP but selectively attenuated morphine-primed reinstatement of CPP, in both mice and rats. Reinstatement of morphine-CPP in mice was accompanied by a selective increase in [3 H]-AMPA binding (but not in [3 H]-MK801 binding) in the ventral hippocampus that was prevented by prior treatment with MLA. Administration of MLA (6.7 μg) directly into the ventral hippocampus of rats prior to a systemic priming dose of morphine abolished reinstatement of morphine-CPP, whereas MLA delivered into the dorsal hippocampus or prefrontal cortex was without effect. These results suggest that α7 nAChRs in the ventral hippocampus play a specific role in the retrieval of associative drug memories following a period of extinction, making them potential targets for the prevention of relapse.
Keywords: [3H]-AMPA; [3H]-MK801; autoradiography; intracerebral drug delivery; methyllycaconitine.
© 2018 The Authors.Addiction Biology published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Conflict of interest statement
The authors do not have any conflicts of interest or financial disclosures to make, related to the data presented in this manuscript.
Figures



References
-
- Addy NA, Nakijama A, Levin E (2003) Nicotinic mechanisms of memory: effects of acute local DHbetaE and MLA infusions in the basolateral amygdala. Brain Res Cogn Brain Res 16:51–57. - PubMed
-
- Aguilar MA, Rodríguez‐Arias M, Miñarro J (2009) Neurobiological mechanisms of the reinstatement of drug‐conditioned place preference. Brain Res Rev 59:253–277. - PubMed
-
- Bardo MT, Bevins RA (2000) Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology 153:31–43. - PubMed
-
- Besson M, David V, Baudonnat M, Cazala P, Guilloux JP, Reperant C, Cloez‐Tayarani I, Changeux JP, Gardier AM, Granon S (2012) Alpha7‐nicotinic receptors modulate nicotine‐induced reinforcement and extracellular dopamine outflow in the mesolimbic system in mice. Psychopharmacology 220:1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

