Strand Displacement Probes Combined with Isothermal Nucleic Acid Amplification for Instrument-Free Detection from Complex Samples
- PMID: 29667809
- PMCID: PMC5990927
- DOI: 10.1021/acs.analchem.8b00269
Strand Displacement Probes Combined with Isothermal Nucleic Acid Amplification for Instrument-Free Detection from Complex Samples
Abstract
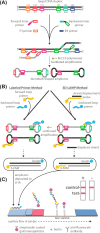
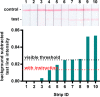
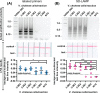
Sensitive and specific detection of pathogens via nucleic acid amplification is currently constrained to laboratory settings and portable equipment with costly fluorescent detectors. Nucleic acid-detecting lateral flow immunoassay strips (LFIAs) offer a low-cost visual transduction strategy at points of need. Unfortunately, these LFIAs frequently detect amplification byproducts that can yield spurious results which can only be deciphered through statistical analysis. We integrated customizable strand displacement probes into standard loop mediated isothermal amplification (LAMP) assays to prevent byproduct capture on commercial LFIAs. We find that combining strand displacement with LAMP (SD-LAMP) yields LFIA test band intensities that can be unequivocally interpreted by human subjects without additional instrumentation, thereby alleviating the need for a portable reader's analysis. Using SD-LAMP, we capture target amplicons on commercially available LFIAs from as few as 3.5 Vibrio cholerae and 2 750 Escherichia coli bacteria without false positive or false negative interpretation. Moreover, we demonstrate that LFIA capture of SD-LAMP products remain specific even in the presence of complex sample matrixes, providing a significant step toward reliable instrument-free pathogen detection outside of laboratories.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Tanner N. A.; Evans T. C. In Current Protocols in Molecular Biology; John Wiley & Sons, Inc., 2014; pp 1–14.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

