Evolution of Genome Architecture in Archaea: Spontaneous Generation of a New Chromosome in Haloferax volcanii
- PMID: 29668953
- PMCID: PMC6063281
- DOI: 10.1093/molbev/msy075
Evolution of Genome Architecture in Archaea: Spontaneous Generation of a New Chromosome in Haloferax volcanii
Abstract
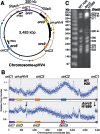
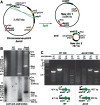
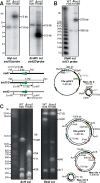
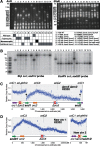
The common ancestry of archaea and eukaryotes is evident in their genome architecture. All eukaryotic and several archaeal genomes consist of multiple chromosomes, each replicated from multiple origins. Three scenarios have been proposed for the evolution of this genome architecture: 1) mutational diversification of a multi-copy chromosome; 2) capture of a new chromosome by horizontal transfer; 3) acquisition of new origins and splitting into two replication-competent chromosomes. We report an example of the third scenario: the multi-origin chromosome of the archaeon Haloferax volcanii has split into two elements via homologous recombination. The newly generated elements are bona fide chromosomes, because each bears "chromosomal" replication origins, rRNA loci, and essential genes. The new chromosomes were stable during routine growth but additional genetic manipulation, which involves selective bottlenecks, provoked further rearrangements. To the best of our knowledge, rearrangement of a naturally evolved prokaryotic genome to generate two new chromosomes has not been described previously.
Figures

References
-
- Beverley SM. 1989. Estimation of circular DNA size using gamma-irradiation and pulsed-field gel electrophoresis. Anal Biochem. 1771:110–114. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

