HITS-CLIP in various brain areas reveals new targets and new modalities of RNA binding by fragile X mental retardation protein
- PMID: 29668986
- PMCID: PMC6158598
- DOI: 10.1093/nar/gky267
HITS-CLIP in various brain areas reveals new targets and new modalities of RNA binding by fragile X mental retardation protein
Abstract
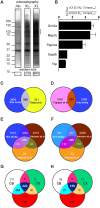
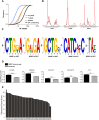
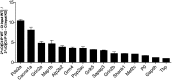
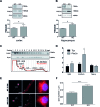
Fragile X syndrome (FXS), the most common form of inherited intellectual disability, is due to the functional deficiency of the fragile X mental retardation protein (FMRP), an RNA-binding protein involved in translational regulation of many messenger RNAs, playing key roles in synaptic morphology and plasticity. To date, no effective treatment for FXS is available. We searched for FMRP targets by HITS-CLIP during early development of multiple mouse brain regions (hippocampus, cortex and cerebellum) at a time of brain development when FMRP is most highly expressed and synaptogenesis reaches a peak. We identified the largest dataset of mRNA targets of FMRP available in brain and we defined their cellular origin. We confirmed the G-quadruplex containing structure as an enriched motif in FMRP RNA targets. In addition to four less represented motifs, our study points out that, in the brain, CTGKA is the prominent motif bound by FMRP, which recognizes it when not engaged in Watson-Crick pairing. All of these motifs negatively modulated the expression level of a reporter protein. While the repertoire of FMRP RNA targets in cerebellum is quite divergent, the ones of cortex and hippocampus are vastly overlapping. In these two brain regions, the Phosphodiesterase 2a (Pde2a) mRNA is a prominent target of FMRP, which modulates its translation and intracellular transport. This enzyme regulates the homeostasis of cAMP and cGMP and represents a novel and attractive therapeutic target to treat FXS.
Figures

References
-
- Maurin T., Zongaro S., Bardoni B.. Fragile X syndrome: from molecular pathology to therapy. Neurosci. Biobehav. Rev. 2014; 46:242–255. - PubMed
-
- van Karnebeek C.D., Bowden K., Berry-Kravis E.. Treatment of neurogenetic developmental conditions: from 2016 into the future. Pediatr. Neurol. 2016; 65:1–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

