Galantamine is not a positive allosteric modulator of human α4β2 or α7 nicotinic acetylcholine receptors
- PMID: 29669164
- PMCID: PMC6016680
- DOI: 10.1111/bph.14329
Galantamine is not a positive allosteric modulator of human α4β2 or α7 nicotinic acetylcholine receptors
Abstract
Background and purpose: The alkaloid galantamine was originally isolated from the green snowdrop Galanthus woronowii and is currently marketed as a drug for treatment of mild to moderate dementia in patients with Alzheimer's disease. In addition to a well-documented proficiency to inhibit acetylcholinesterase, galantamine has been reported to increase neuronal nicotinic ACh (nACh) receptor function by acting as a positive allosteric modulator. Yet there remains controversy regarding these findings in the literature. To resolve this conundrum, we evaluated galantamine actions at α4β2 and α7, which represent the nACh receptors most commonly associated with mammalian cognitive domains.
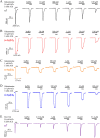
Experimental approach: α4β2 [in (α4)3 (β2)2 and (α4)2 (β2)3 stoichiometries] and α7 nACh receptors were expressed in Xenopus laevis oocytes and subjected to two-electrode voltage-clamp electrophysiological experiments. Galantamine (10 nM to 100 μM) was evaluated for direct agonist effects and for positive modulation by co-application with sub-maximally efficacious concentrations of ACh. In addition, similar experiments were performed with α7 nACh receptors stably expressed in HEK293 cells using patch-clamp electrophysiology.
Key results: In concentrations ranging from 10 nM to 1 μM, galantamine did not display direct agonism nor positive modulatory effects at any receptor combination tested. At concentrations from 10 μM and above, galantamine inhibited the activity with a mechanism of action consistent with open-channel pore blockade at all receptor types.
Conclusion and implications: Based on our data, we conclude that galantamine is not a positive allosteric modulator of α7 or α4β2 receptors, which represent the majority of nACh receptors in mammalian brain.
© 2018 The British Pharmacological Society.
Figures





Similar articles
-
Effect of galantamine on the human alpha7 neuronal nicotinic acetylcholine receptor, the Torpedo nicotinic acetylcholine receptor and spontaneous cholinergic synaptic activity.Br J Pharmacol. 2005 Jul;145(5):672-8. doi: 10.1038/sj.bjp.0706221. Br J Pharmacol. 2005. PMID: 15834443 Free PMC article.
-
Augmentation of cognitive function by NS9283, a stoichiometry-dependent positive allosteric modulator of α2- and α4-containing nicotinic acetylcholine receptors.Br J Pharmacol. 2012 Sep;167(1):164-82. doi: 10.1111/j.1476-5381.2012.01989.x. Br J Pharmacol. 2012. PMID: 22506660 Free PMC article.
-
AE Succinimide, an Analogue of Methyllycaconitine, When Bound Generates a Nonconducting Conformation of the α4β2 Nicotinic Acetylcholine Receptor.ACS Chem Neurosci. 2020 Feb 5;11(3):344-355. doi: 10.1021/acschemneuro.9b00525. Epub 2020 Jan 14. ACS Chem Neurosci. 2020. PMID: 31898891
-
Nicotinic cholinergic modulation: galantamine as a prototype.CNS Drug Rev. 2002 Winter;8(4):405-26. doi: 10.1111/j.1527-3458.2002.tb00237.x. CNS Drug Rev. 2002. PMID: 12481195 Free PMC article. Review.
-
Cholinesterase inhibitors used in the treatment of Alzheimer's disease: the relationship between pharmacological effects and clinical efficacy.Drugs Aging. 2004;21(7):453-78. doi: 10.2165/00002512-200421070-00004. Drugs Aging. 2004. PMID: 15132713 Review.
Cited by
-
Radiosynthesis, Preclinical, and Clinical Positron Emission Tomography Studies of Carbon-11 Labeled Endogenous and Natural Exogenous Compounds.Chem Rev. 2023 Jan 11;123(1):105-229. doi: 10.1021/acs.chemrev.2c00398. Epub 2022 Nov 18. Chem Rev. 2023. PMID: 36399832 Free PMC article. Review.
-
Impact of modulation of the α7 nicotinic acetylcholine receptor on nicotine reward in the mouse conditioned place preference test.Psychopharmacology (Berl). 2019 Dec;236(12):3593-3599. doi: 10.1007/s00213-019-05331-y. Epub 2019 Jul 13. Psychopharmacology (Berl). 2019. PMID: 31302720 Free PMC article.
-
Galantamine-Memantine Combination in the Treatment of Parkinson's Disease Dementia.Brain Sci. 2024 Nov 21;14(12):1163. doi: 10.3390/brainsci14121163. Brain Sci. 2024. PMID: 39766362 Free PMC article. Review.
-
Auditory mismatch responses are differentially sensitive to changes in muscarinic acetylcholine versus dopamine receptor function.Elife. 2022 May 3;11:e74835. doi: 10.7554/eLife.74835. Elife. 2022. PMID: 35502897 Free PMC article. Clinical Trial.
-
Concatenated γ-aminobutyric acid type A receptors revisited: Finding order in chaos.J Gen Physiol. 2019 Jun 3;151(6):798-819. doi: 10.1085/jgp.201812133. Epub 2019 Apr 15. J Gen Physiol. 2019. PMID: 30988061 Free PMC article.
References
-
- Ago Y, Koda K, Takuma K, Matsuda T (2011). Pharmacological aspects of the acetylcholinesterase inhibitor galantamine. J Pharmacol Sci 116: 6–17. - PubMed
-
- Ahring PK, Bang LH, Jensen ML, Strøbæk D, Hartiadi LY, Chebib M et al (2016). A pharmacological assessment of agonists and modulators at α4β2γ2 and α4β2δ GABAA receptors: the challenge in comparing apples with oranges. Pharmacol Res 111: 563–576. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

