Blending genomes: distributive conjugal transfer in mycobacteria, a sexier form of HGT
- PMID: 29669186
- PMCID: PMC5997560
- DOI: 10.1111/mmi.13971
Blending genomes: distributive conjugal transfer in mycobacteria, a sexier form of HGT
Abstract
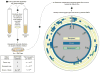
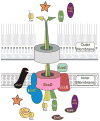
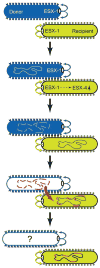
This review discusses a novel form of horizontal gene transfer (HGT) found in mycobacteria called Distributive Conjugal Transfer (DCT). While satisfying the criteria for conjugation, DCT occurs by a mechanism so distinct from oriT-mediated conjugation that it could be considered a fourth category of HGT. DCT involves the transfer of chromosomal DNA between mycobacteria and, most significantly, generates transconjugants with mosaic genomes of the parental strains. Multiple segments of donor chromosomal DNA can be co-transferred regardless of their location or the genetic selection and, as a result, the transconjugant genome contains many donor-derived segments; hence the name DCT. This distinguishing feature of DCT separates it from the other known mechanisms of HGT, which generally result in the introduction of a single, defined segment of DNA into the recipient chromosome (Fig. ). Moreover, these mosaic progeny are generated from a single conjugal event, which provides enormous capacity for rapid adaptation and evolution, again distinguishing it from the three classical modes of HGT. Unsurprisingly, the unusual mosaic products of DCT are generated by a conjugal mechanism that is also unusual. Here, we will describe the unique features of DCT and contrast those to other mechanisms of HGT, both from a mechanistic and an evolutionary perspective. Our focus will be on transfer of chromosomal DNA, as opposed to plasmid mobilization, because DCT mediates transfer of chromosomal DNA and is a chromosomally encoded process.
© 2018 John Wiley & Sons Ltd.
Figures

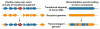
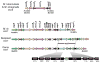
Similar articles
-
Distributive Conjugal Transfer: New Insights into Horizontal Gene Transfer and Genetic Exchange in Mycobacteria.Microbiol Spectr. 2014 Feb;2(1):MGM2-0022-2013. doi: 10.1128/microbiolspec.MGM2-0022-2013. Microbiol Spectr. 2014. PMID: 26082110 Review.
-
Quantifying and Characterizing Distributive Conjugal Transfer in Mycobacterium smegmatis.Methods Mol Biol. 2020;2075:123-134. doi: 10.1007/978-1-4939-9877-7_9. Methods Mol Biol. 2020. PMID: 31584159
-
A Polymorphic Gene within the Mycobacterium smegmatis esx1 Locus Determines Mycobacterial Self-Identity and Conjugal Compatibility.mBio. 2022 Apr 26;13(2):e0021322. doi: 10.1128/mbio.00213-22. Epub 2022 Mar 17. mBio. 2022. PMID: 35297678 Free PMC article.
-
Distributive Conjugal Transfer: New Insights into Horizontal Gene Transfer and Genetic Exchange in Mycobacteria.Microbiol Spectr. 2014;2(1):04. doi: 10.1128/microbiolspec.MGM2-0022-2013. Microbiol Spectr. 2014. PMID: 25505644 Free PMC article.
-
Horizontal transfer of antibiotic resistance genes in clinical environments.Can J Microbiol. 2019 Jan;65(1):34-44. doi: 10.1139/cjm-2018-0275. Epub 2018 Sep 24. Can J Microbiol. 2019. PMID: 30248271 Review.
Cited by
-
The PulE ATPase is required for twitching motility and DNA donation during Thermus thermophilus transjugation.Int Microbiol. 2025 Jun;28(5):1047-1055. doi: 10.1007/s10123-024-00598-4. Epub 2024 Sep 26. Int Microbiol. 2025. PMID: 39325340
-
Population genomics provides insights into the evolution and adaptation to humans of the waterborne pathogen Mycobacterium kansasii.Nat Commun. 2021 May 3;12(1):2491. doi: 10.1038/s41467-021-22760-6. Nat Commun. 2021. PMID: 33941780 Free PMC article.
-
Multidrug Resistance (MDR) and Collateral Sensitivity in Bacteria, with Special Attention to Genetic and Evolutionary Aspects and to the Perspectives of Antimicrobial Peptides-A Review.Pathogens. 2020 Jun 29;9(7):522. doi: 10.3390/pathogens9070522. Pathogens. 2020. PMID: 32610480 Free PMC article. Review.
-
Direct cell-cell contact activates SigM to express the ESX-4 secretion system in Mycobacterium smegmatis.Proc Natl Acad Sci U S A. 2018 Jul 10;115(28):E6595-E6603. doi: 10.1073/pnas.1804227115. Epub 2018 Jun 25. Proc Natl Acad Sci U S A. 2018. PMID: 29941598 Free PMC article.
-
The Flp type IV pilus operon of Mycobacterium tuberculosis is expressed upon interaction with macrophages and alveolar epithelial cells.Front Cell Infect Microbiol. 2022 Sep 20;12:916247. doi: 10.3389/fcimb.2022.916247. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36204636 Free PMC article.
References
-
- Abdallah AM, Gey van Pittius NC, DiGiuseppe Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CMJE, Appelmelk BJ, Bitter W. Type VII secretion — mycobacteria show the way. Nature Reviews Microbiology. 2007;5:883–891. - PubMed
-
- Abdallah AM, Verboom T, Hannes F, Safi M, Strong M, Eisenberg D, Musters RJP, Vandenbroucke-Grauls CMJE, Appelmelk BJ, Luirink J, Bitter W. A specific secretion system mediates PPE41 transport in pathogenic mycobacteria. Molecular Microbiology. 2006;62:667–679. - PubMed
-
- Abdallah AM, Verboom T, Weerdenburg EM, Gey van Pittius NC, Mahasha PW, Jiménez C, Parra M, Cadieux N, Brennan MJ, Appelmelk BJ, Bitter W. PPE and PE_PGRS proteins of Mycobacterium marinum are transported via the type VII secretion system ESX-5. Molecular Microbiology. 2009;73:329–340. - PubMed
-
- Ates LS, Houben EN, Bitter W. Type VII Secretion: A Highly Versatile Secretion System. Microbiol Spectr. 2016a:4. - PubMed
-
- Ates LS, van der Woude AD, Bestebroer J, van Stempvoort G, Musters RJP, Garcia-Vallejo JJ, Picavet DI, Weerd Rvd, Maletta M, Kuijl CP, van der Wel NN, Bitter W. The ESX-5 System of Pathogenic Mycobacteria Is Involved In Capsule Integrity and Virulence through Its Substrate PPE10. PLoS Pathog. 2016b;12:e1005696. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

