Self-Renewal and Toll-like Receptor Signaling Sustain Exhausted Plasmacytoid Dendritic Cells during Chronic Viral Infection
- PMID: 29669251
- PMCID: PMC5937984
- DOI: 10.1016/j.immuni.2018.03.020
Self-Renewal and Toll-like Receptor Signaling Sustain Exhausted Plasmacytoid Dendritic Cells during Chronic Viral Infection
Abstract
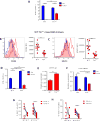
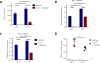
Although characterization of T cell exhaustion has unlocked powerful immunotherapies, the mechanisms sustaining adaptations of short-lived innate cells to chronic inflammatory settings remain unknown. During murine chronic viral infection, we found that concerted events in bone marrow and spleen mediated by type I interferon (IFN-I) and Toll-like receptor 7 (TLR7) maintained a pool of functionally exhausted plasmacytoid dendritic cells (pDCs). In the bone marrow, IFN-I compromised the number and the developmental capacity of pDC progenitors, which generated dysfunctional pDCs. Concurrently, exhausted pDCs in the periphery were maintained by self-renewal via IFN-I- and TLR7-induced proliferation of CD4- subsets. On the other hand, pDC functional loss was mediated by TLR7, leading to compromised IFN-I production and resistance to secondary infection. These findings unveil the mechanisms sustaining a self-perpetuating pool of functionally exhausted pDCs and provide a framework for deciphering long-term exhaustion of other short-lived innate cells during chronic inflammation.
Keywords: HIV; LCMV; TLR; cancer; chronic viral infection; exhaustion; plasmacytoid dendritic cells; type I interferon.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures

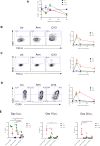
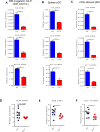
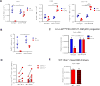
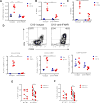
Similar articles
-
Interleukin-27R Signaling Mediates Early Viral Containment and Impacts Innate and Adaptive Immunity after Chronic Lymphocytic Choriomeningitis Virus Infection.J Virol. 2018 May 29;92(12):e02196-17. doi: 10.1128/JVI.02196-17. Print 2018 Jun 15. J Virol. 2018. PMID: 29593047 Free PMC article.
-
Hematopoietic and nonhematopoietic cells promote Type I interferon- and TLR7-dependent monocytosis during low-dose LCMV infection.Eur J Immunol. 2015 Nov;45(11):3064-72. doi: 10.1002/eji.201445331. Epub 2015 Sep 14. Eur J Immunol. 2015. PMID: 26289159 Free PMC article.
-
Viral infection switches non-plasmacytoid dendritic cells into high interferon producers.Nature. 2003 Jul 17;424(6946):324-8. doi: 10.1038/nature01783. Epub 2003 Jun 22. Nature. 2003. PMID: 12819664
-
[Toll-like receptors that sense viral infection].Uirusu. 2004 Jun;54(1):1-8. doi: 10.2222/jsv.54.1. Uirusu. 2004. PMID: 15449898 Review. Japanese.
-
Sex Differences in Primary HIV Infection: Revisiting the Role of TLR7-Driven Type 1 IFN Production by Plasmacytoid Dendritic Cells in Women.Front Immunol. 2021 Aug 27;12:729233. doi: 10.3389/fimmu.2021.729233. eCollection 2021. Front Immunol. 2021. PMID: 34512664 Free PMC article. Review.
Cited by
-
Regulation and function of Id2 in plasmacytoid dendritic cells.Mol Immunol. 2022 Aug;148:6-17. doi: 10.1016/j.molimm.2022.05.009. Epub 2022 May 28. Mol Immunol. 2022. PMID: 35640521 Free PMC article.
-
Emerging concepts of type I interferons in SLE pathogenesis and therapy.Nat Rev Rheumatol. 2022 Oct;18(10):575-590. doi: 10.1038/s41584-022-00826-z. Epub 2022 Sep 12. Nat Rev Rheumatol. 2022. PMID: 36097207 Review.
-
Infection and cancer suppress pDC derived IFN-I.Curr Opin Immunol. 2020 Oct;66:114-122. doi: 10.1016/j.coi.2020.08.001. Epub 2020 Sep 15. Curr Opin Immunol. 2020. PMID: 32947131 Free PMC article. Review.
-
IRF5 genetic risk variants drive myeloid-specific IRF5 hyperactivation and presymptomatic SLE.JCI Insight. 2020 Jan 30;5(2):e124020. doi: 10.1172/jci.insight.124020. JCI Insight. 2020. PMID: 31877114 Free PMC article.
-
Regulation of Transcription Factor E2-2 in Human Plasmacytoid Dendritic Cells by Monocyte-Derived TNFα.Viruses. 2020 Jan 31;12(2):162. doi: 10.3390/v12020162. Viruses. 2020. PMID: 32023836 Free PMC article.
References
-
- Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–6477. - PubMed
-
- Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

