Three-Dimensional Human iPSC-Derived Artificial Skeletal Muscles Model Muscular Dystrophies and Enable Multilineage Tissue Engineering
- PMID: 29669293
- PMCID: PMC5917451
- DOI: 10.1016/j.celrep.2018.03.091
Three-Dimensional Human iPSC-Derived Artificial Skeletal Muscles Model Muscular Dystrophies and Enable Multilineage Tissue Engineering
Abstract
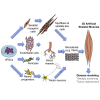
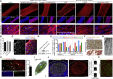
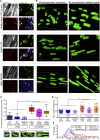
Generating human skeletal muscle models is instrumental for investigating muscle pathology and therapy. Here, we report the generation of three-dimensional (3D) artificial skeletal muscle tissue from human pluripotent stem cells, including induced pluripotent stem cells (iPSCs) from patients with Duchenne, limb-girdle, and congenital muscular dystrophies. 3D skeletal myogenic differentiation of pluripotent cells was induced within hydrogels under tension to provide myofiber alignment. Artificial muscles recapitulated characteristics of human skeletal muscle tissue and could be implanted into immunodeficient mice. Pathological cellular hallmarks of incurable forms of severe muscular dystrophy could be modeled with high fidelity using this 3D platform. Finally, we show generation of fully human iPSC-derived, complex, multilineage muscle models containing key isogenic cellular constituents of skeletal muscle, including vascular endothelial cells, pericytes, and motor neurons. These results lay the foundation for a human skeletal muscle organoid-like platform for disease modeling, regenerative medicine, and therapy development.
Keywords: disease modeling; iPS cells; muscular dystrophy; myogenic differentiation; organoids; pluripotent stem cells; skeletal muscle; tissue engineering.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Armulik A., Genové G., Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell. 2011;21:193–215. - PubMed
-
- Bertrand A.T., Ziaei S., Ehret C., Duchemin H., Mamchaoui K., Bigot A., Mayer M., Quijano-Roy S., Desguerre I., Lainé J. Cellular microenvironments reveal defective mechanosensing responses and elevated YAP signaling in LMNA-mutated muscle precursors. J. Cell Sci. 2014;127:2873–2884. - PubMed
-
- Chal J., Oginuma M., Al Tanoury Z., Gobert B., Sumara O., Hick A., Bousson F., Zidouni Y., Mursch C., Moncuquet P. Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat. Biotechnol. 2015;33:962–969. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

