Assessment of an optimized manufacturing process for inactivated quadrivalent influenza vaccine: a phase III, randomized, double-blind, safety and immunogenicity study in children and adults
- PMID: 29669531
- PMCID: PMC5907359
- DOI: 10.1186/s12879-018-3079-8
Assessment of an optimized manufacturing process for inactivated quadrivalent influenza vaccine: a phase III, randomized, double-blind, safety and immunogenicity study in children and adults
Abstract
Background: GSK has modified the licensed monovalent bulk manufacturing process for its split-virion inactivated quadrivalent influenza vaccine (IIV4) to harmonize the process among different strains, resulting in an increased number of finished vaccine doses, while compensating for the change from inactivated trivalent influenza vaccine (IIV3) to IIV4. To confirm the manufacturing changes do not alter the profile of the vaccine, a clinical trial was conducted to compare IIV4 made by the currently licensed process with a vaccine made by the new (investigational) process (IIV4-I). The main objectives were to compare the reactogenicity and safety of IIV4-I versus IIV4 in all age groups, and to demonstrate the non-inferiority of the hemagglutination-inhibition (HI) antibody responses based on the geometric mean titer ratio of IIV4-I versus IIV4 in children.
Methods: The Phase III, randomized, double-blind, multinational study included three cohorts: adults (18-49 years; N = 120), children (3-17 years; N = 821), and infants (6-35 months; N = 940). Eligible subjects in each cohort were randomized 1:1 to receive IIV4-I or IIV4. Both vaccines contained 15 μg of hemagglutinin antigen for each of the four seasonal virus strains. Adults and vaccine-primed children received one dose of vaccine, and vaccine-unprimed children received two doses of vaccine 28 days apart. All children aged ≥9 years were considered to be vaccine-primed and received one dose of vaccine.
Results: The primary immunogenicity objective of the study was met in demonstrating immunogenic non-inferiority of IIV4-I versus IIV4 in children. The IIV4-I was immunogenic against all four vaccine strains in each age cohort. The reactogenicity and safety profile of IIV4-I was similar to IIV4 in each age cohort, and there was no increase in the relative risk of fever (≥38 °C) with IIV4-I versus IIV4 within the 7-day post-vaccination period in infants (1.06; 95% Confidence Interval: 0.75, 1.50; p = 0.786).
Conclusions: The study demonstrated that in adults, children, and infants, the IIV4-I made using an investigational manufacturing process was immunogenic with a reactogenicity and safety profile that was similar to licensed IIV4. These results support that the investigational process used to manufacture IIV4-I is suitable to replace the current licensed process.
Trial registration: ClinicalTrials.gov: NCT02207413 ; trial registration date: August 4, 2014.
Keywords: Adults; Children; Immunogenicity; Infants; Influenza vaccine; Investigational; Manufacturing; Quadrivalent; Reactogenicity; Safety.
Conflict of interest statement
Ethics approval and consent to participate
All adults or a parent/guardian of children provided written informed consent for participation, and children were required to assent if capable. The study was conducted in accordance with the Good Clinical Practice guidelines, the Declaration of Helsinki and applicable local regulations. All study documents were approved by the appropriate Institutional Review Boards (Bangladesh: icddr,b; Czech Republic: Eticka komise Fakultni nemocnice Olomouc a Lekarske fakulty UP v Olomouci [68/14 MEK 9]; France: CPP SUD MEDITERANEE V [14.045]; Germany: Ethik-Kommission der Medizinischen Fakultät der Julius-Maximilians-Universität Würzburg [129/14 ff]; Poland: Komisja Bioetyczna przy Okregowej Izbie Lekarskiej; Spain: Comite Etico de Investigacion Clínica Area de Salud de Burgos y Soria; United States: Chesapeake IRB).
Competing interests
Bruce L. Innis, Varsha K. Jain and Ping Li were employed by the GSK group of companies at the time of the study. Bruce L. Innis is currently employed by PATH. Varsha K. Jain is currently employed by the Bill and Melinda Gates Foundation. Ping Li is currently employed by Pfizer. Carine Claeys, Mamadou Drame, Peter Schu and Elisabeth Neumeier are employed by the GSK group of companies. Carine Claeys, Peter Schu, Elisabeth Neumeier, Ping Li, Varsha K. Jain, Bruce L. Innis hold shares in the GSK group of companies. Federico Martinón-Torres reports investigator fees to his institution from the GSK group of companies, during the conduct of the study; grants to his institution from the GSK group of companies, SPMSD, MERCK and PFIZER, as well as personal fees from PFIZER, outside the submitted work. Franck Thollot reports personal fees from the GSK group of companies, during the conduct of the study; personal fees from the GSK group of companies and non-financial support from Sanofi Aventis, outside the submitted work. Michael Horn reports personal fees from the GSK group of companies, during the conduct of the study. Tino F. Schwarz reports personal fees from the GSK group of companies, outside the submitted work. José M. Merino reports personal fees and non-financial support from the GSK group of companies, during the conduct of the study; personal fees from PFIZER and SANOFI-PASTEUR, outside the submitted work. José García-Sicilia reports fees to his institution from the GSK group of companies, during the conduct of the study. Khalequ Zaman, Alfonso Carmona, Phu My Tran, Mariano Miranda, Ulrich Behre, Iwona Sadowska-Krawczenko and Henryk Szymański declare no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

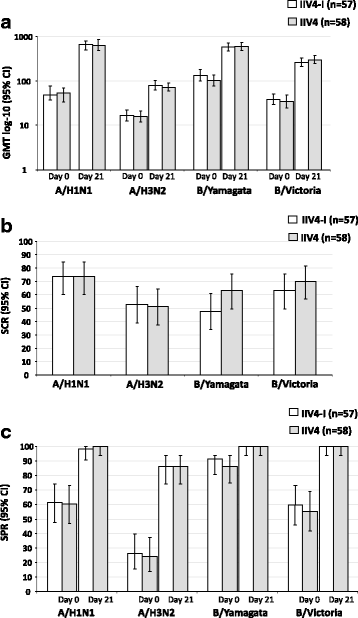



References
-
- United States Centers for Disease Control and Prevention.: Seasonal influenza activity surveillance reports: 2000-2001 to 2010-2011 seasons. http://www.cdc.gov/flu/weekly/pastreports.htm. Accessed August 2017.
-
- Belongia EA, Kieke BA, Donahue JG, Greenlee RT, Balish A, Foust A, Lindstrom S, Shay DK. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004-2005 season to the 2006-2007 season. J Infect Dis. 2009;199(2):159–167. doi: 10.1086/595861. - DOI - PubMed
-
- World Health Organization.: Recommended composition of influenza virus vaccines for use in the 2012-2013 northern hemisphere influenza season. 2012, http://www.who.int/influenza/vaccines/virus/recommendations/201202_recom.... Accessed August 2017.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

