Misoprostol administered sublingually at a dose of 12.5 μg versus vaginally at a dose of 25 μg for the induction of full-term labor: a randomized controlled trial protocol
- PMID: 29669596
- PMCID: PMC5907413
- DOI: 10.1186/s12978-018-0508-5
Misoprostol administered sublingually at a dose of 12.5 μg versus vaginally at a dose of 25 μg for the induction of full-term labor: a randomized controlled trial protocol
Abstract
Background: Various methods are currently used for the induction of labor. Nevertheless, the most effective method with the fewest side effects remains to be established. Misoprostol, administered vaginally, has been routinely used for this purpose; however, other forms of administration are being proposed, including the use of sublingual tablets. No studies have yet compared the effectiveness and safety of 12.5-μg misoprostol administered sublingually compared to a 25-μg vaginal dose of the drug for the induction of labor.
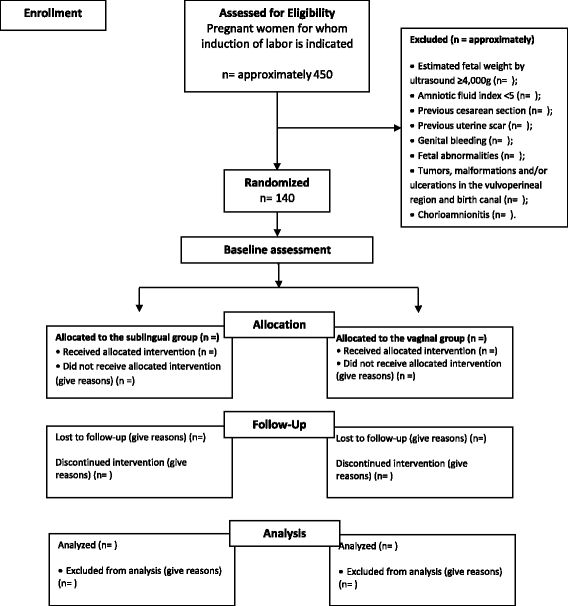
Methods: A triple-blind, multicenter, placebo-controlled, randomized clinical trial will be conducted in Brazil at the Instituto de Medicina Integral Prof. Fernando Figueira and at the Assis Chateaubriand Maternity Teaching Hospital of the Federal University of Ceará. A total of 140 patients with full-term pregnancies, a live fetus, a Bishop score ≤ 6 and a recommendation of induction of labor will be randomized to one of two groups. One group will receive 12.5-μg sublingual tablets of misoprostol and placebo vaginal tablets, while the other group will receive placebo sublingual tablets and vaginal tablets containing 25 μg of misoprostol. The principal endpoint is the rate of tachysystole. The secondary endpoints are vaginal delivery within 24 h of induction, uterine hyperstimulation, Cesarean section, severe neonatal morbidity or perinatal death, severe maternal morbidity or maternal death, and maternal preference regarding the route of administration of the drug. Student's t-test, and the chi-square test of association or Fisher's exact test, as appropriate, will be used in the data analysis. Risk ratios and their respective 95% confidence intervals will be calculated.
Discussion: Misoprostol has been identified as a safe, inexpensive, easily administered option for the induction of labor, with satisfactory results. An experimental study has shown that misoprostol administered sublingually at a dose of 25 μg appears to be effective and is associated with greater maternal satisfaction when labor is induced in women with an unfavorable cervix. Nevertheless, the rate of tachysystole remains high; therefore, further studies are required to determine the ideal dose and the ideal interval of time between doses.
Trial registration: ClinicalTrial.gov, NCT01406392 .
Keywords: Clinical trial; Labor, induced; Labor, obstetric; Misoprostol/administration & dosage administration, sublingual; Multicenter study.
Conflict of interest statement
Ethics approval and consent to participate
The research project was APPROVED by the Ethics Committee on Research in Human Beings of the Instituto de Medicina Integral Professor Fernando Figueira (IMIP), Brazil (number 2137–11) and the Ethics Committee of the Maternity-School Assis Chateaubriand of the Federal University of Ceará, Brazil (Protocol 59/11). All participants will sign an informed consent form prior to their admission to the study. A steering committee has been formed to monitor the trial.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Moraes Filho OB, Cecatti JG, FEL F. Métodos para indução do parto. Rev Bras Ginecol Obs. 2005;27:493–500. doi: 10.1590/S0100-72032005000800010. - DOI
-
- Margulies M, Voto LS, Catuzzi PIF. Inducción del trabajo de parto con un análogo de la PGE1. Prensa Med Arg. 1991;78:9–13.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical