Modulation of the secretory pathway by amino-acid starvation
- PMID: 29669743
- PMCID: PMC6028531
- DOI: 10.1083/jcb.201802003
Modulation of the secretory pathway by amino-acid starvation
Abstract
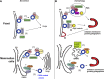
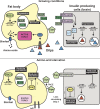
As a major anabolic pathway, the secretory pathway needs to adapt to the demands of the surrounding environment and responds to different exogenous signals and stimuli. In this context, the transport in the early secretory pathway from the endoplasmic reticulum (ER) to the Golgi apparatus appears particularly regulated. For instance, protein export from the ER is critically stimulated by growth factors. Conversely, nutrient starvation also modulates functions of the early secretory pathway in multiple ways. In this review, we focus on amino-acid starvation and how the function of the early secretory pathway is redirected to fuel autophagy, how the ER exit sites are remodeled into novel cytoprotective stress assemblies, and how secretion is modulated in vivo in starving organisms. With the increasingly exciting knowledge on mechanistic target of rapamycin complex 1 (mTORC1), the major nutrient sensor, it is also a good moment to establish how the modulation of the secretory pathway by amino-acid restriction intersects with this major signaling hub.
© 2018 van Leeuwen et al.
Figures

References
-
- Aguilera-Gomez A., Zacharogianni M., van Oorschot M.M., Genau H., Grond R., Veenendaal T., Sinsimer K.S., Gavis E.R., Behrends C., and Rabouille C.. 2017. Phospho-Rasputin Stabilization by Sec16 Is Required for Stress Granule Formation upon Amino Acid Starvation. Cell Reports. 20:935–948. 10.1016/j.celrep.2017.06.042 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

