Single cardiac ventricular myosins are autonomous motors
- PMID: 29669825
- PMCID: PMC5936712
- DOI: 10.1098/rsob.170240
Single cardiac ventricular myosins are autonomous motors
Abstract
Myosin transduces ATP free energy into mechanical work in muscle. Cardiac muscle has dynamically wide-ranging power demands on the motor as the muscle changes modes in a heartbeat from relaxation, via auxotonic shortening, to isometric contraction. The cardiac power output modulation mechanism is explored in vitro by assessing single cardiac myosin step-size selection versus load. Transgenic mice express human ventricular essential light chain (ELC) in wild- type (WT), or hypertrophic cardiomyopathy-linked mutant forms, A57G or E143K, in a background of mouse α-cardiac myosin heavy chain. Ensemble motility and single myosin mechanical characteristics are consistent with an A57G that impairs ELC N-terminus actin binding and an E143K that impairs lever-arm stability, while both species down-shift average step-size with increasing load. Cardiac myosin in vivo down-shifts velocity/force ratio with increasing load by changed unitary step-size selections. Here, the loaded in vitro single myosin assay indicates quantitative complementarity with the in vivo mechanism. Both have two embedded regulatory transitions, one inhibiting ADP release and a second novel mechanism inhibiting actin detachment via strain on the actin-bound ELC N-terminus. Competing regulators filter unitary step-size selection to control force-velocity modulation without myosin integration into muscle. Cardiac myosin is muscle in a molecule.
Keywords: Qdot labelled actin under load; cardiomyopathy-linked mutants; ratcheting myosin essential light chain; single cardiac myosin mechanics; super-resolution microscopy.
© 2018 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures


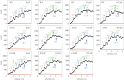
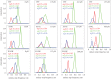
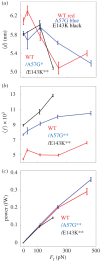
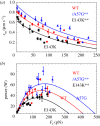
 (b) average duty ratio and (c) power for WT (red), A57G (blue) and E143K (black) αcardiac myosin measured by the Qdot assay under a retarding force. Data points are derived from simulation of the corresponding event–velocity histograms in figure 3 and as described in ‘Qdot assay event–velocity histogram simulation’. Errors are standard error of the mean for the 16–55 replicates described in ‘Statistics’. Data in pairwise comparison for the WT and mutant species are indicated for each panel. They differ significantly with confidence level p < 0.05 or 0.01 indicated by * or **.
(b) average duty ratio and (c) power for WT (red), A57G (blue) and E143K (black) αcardiac myosin measured by the Qdot assay under a retarding force. Data points are derived from simulation of the corresponding event–velocity histograms in figure 3 and as described in ‘Qdot assay event–velocity histogram simulation’. Errors are standard error of the mean for the 16–55 replicates described in ‘Statistics’. Data in pairwise comparison for the WT and mutant species are indicated for each panel. They differ significantly with confidence level p < 0.05 or 0.01 indicated by * or **.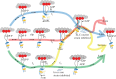



References
-
- Geeves MA, Fedorov R, Manstein DJ. 2005. Molecular mechanism of actomyosin-based motility. Cell. Mol. Life Sci. 62, 1462–1477. (doi:10.1007/s00018-005-5015-5) - DOI - PMC - PubMed
-
- Huxley AF. 1957. Muscle structure and theories of contraction. Prog. Biophys. Chem. 7, 255–318. - PubMed
-
- Rayment I, Holden HM. 1994. The three-dimensional structure of a molecular motor. Trends Biochem. Sci. 19, 129–134. (doi:10.1016/0968-0004(94)90206-2) - DOI - PubMed
-
- Sherwood JJ, Waller GS, Warshaw DM, Lowey S. 2004. A point mutation in the regulatory light chain reduces the step size of skeletal muscle myosin. Proc. Natl Acad. Sci. USA 101, 10 973–10 978. (doi:10.1073/pnas.0401699101) - DOI - PMC - PubMed
-
- Steffen W, Smith D, Simmons R, Sleep J. 2001. Mapping the actin filament with myosin. Proc. Natl Acad. Sci. USA 98, 14 949–14 954. (doi:10.1073/pnas.261560698) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

