CD32 is expressed on cells with transcriptionally active HIV but does not enrich for HIV DNA in resting T cells
- PMID: 29669853
- PMCID: PMC6282755
- DOI: 10.1126/scitranslmed.aar6759
CD32 is expressed on cells with transcriptionally active HIV but does not enrich for HIV DNA in resting T cells
Abstract
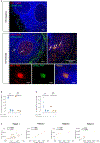
The persistence of HIV reservoirs, including latently infected, resting CD4+ T cells, is the major obstacle to cure HIV infection. CD32a expression was recently reported to mark CD4+ T cells harboring a replication-competent HIV reservoir during antiretroviral therapy (ART) suppression. We aimed to determine whether CD32 expression marks HIV latently or transcriptionally active infected CD4+ T cells. Using peripheral blood and lymphoid tissue of ART-treated HIV+ or SIV+ subjects, we found that most of the circulating memory CD32+ CD4+ T cells expressed markers of activation, including CD69, HLA-DR, CD25, CD38, and Ki67, and bore a TH2 phenotype as defined by CXCR3, CCR4, and CCR6. CD32 expression did not selectively enrich for HIV- or SIV-infected CD4+ T cells in peripheral blood or lymphoid tissue; isolated CD32+ resting CD4+ T cells accounted for less than 3% of the total HIV DNA in CD4+ T cells. Cell-associated HIV DNA and RNA loads in CD4+ T cells positively correlated with the frequency of CD32+ CD69+ CD4+ T cells but not with CD32 expression on resting CD4+ T cells. Using RNA fluorescence in situ hybridization, CD32 coexpression with HIV RNA or p24 was detected after in vitro HIV infection (peripheral blood mononuclear cell and tissue) and in vivo within lymph node tissue from HIV-infected individuals. Together, these results indicate that CD32 is not a marker of resting CD4+ T cells or of enriched HIV DNA-positive cells after ART; rather, CD32 is predominately expressed on a subset of activated CD4+ T cells enriched for transcriptionally active HIV after long-term ART.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures





References
-
- Wong JK, Hezareh M, Günthard HF, Havlir DV, Ignacio CC, Spina CA,Richman DD, Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278, 1291–1295 (1997). - PubMed
-
- Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, Deeks SG,Luciw PA, Chipman JG, Beilman GJ, Hoskuldsson T, Khoruts A, Anderson J,Deleage C, Jasurda J, Schmidt TE, Hafertepe M, Callisto SP, Pearson H, Reimann T, Schuster J, Schoephoerster J, Southern P, Perkey K, Shang L, Wietgrefe SW,Fletcher CV, Lifson JD, Douek DC, McCune JM, Haase AT, Schacker TW, Defining total-body AIDS-virus burden with implications for curative strategies. Nat. Med 23, 1271–1276 (2017). - PMC - PubMed
-
- Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R,Deleage C, Lucero C, Morcock D, Swanson T, Legasse AW, Axthelm MK,Hesselgesser J, Geleziunas R, Hirsch VM, Edlefsen PT, Piatak M Jr., Estes JD, Lifson JD, Picker LJ, B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med 21, 132–139 (2015). - PMC - PubMed
-
- McGary CS, Deleage C, Harper J, Micci L, Ribeiro SP, Paganini S, Kuri-Cervantes L,Benne C, Ryan ES, Balderas R, Jean S, Easley K, Marconi V, Silvestri G, Estes JD,Sekaly RP, Paiardini M, CTLA-4+PD-1− memory CD4+ T cells critically contribute to viral persistence in antiretroviral therapy-suppressed, SIV-infected rhesus macaques. Immunity 47, 776–788.e5 (2017). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI126620/AI/NIAID NIH HHS/United States
- P30 AI050409/AI/NIAID NIH HHS/United States
- R21 NS106970/NS/NINDS NIH HHS/United States
- R01 AI124843/AI/NIAID NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- UM1 AI126619/AI/NIAID NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- R01 AI118694/AI/NIAID NIH HHS/United States
- R21 AI118411/AI/NIAID NIH HHS/United States
- R21 AI129636/AI/NIAID NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
- UL1 TR002538/TR/NCATS NIH HHS/United States
- P30 CA010815/CA/NCI NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- U01 AI065279/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

