Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection
- PMID: 29669922
- PMCID: PMC5939091
- DOI: 10.1073/pnas.1721345115
Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection
Abstract
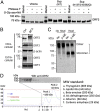
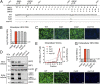
The enterically transmitted hepatitis E virus (HEV) adopts a unique strategy to exit cells by cloaking its capsid (encoded by the viral ORF2 gene) and circulating in the blood as "quasi-enveloped" particles. However, recent evidence suggests that the majority of the ORF2 protein present in the patient serum and supernatants of HEV-infected cell culture exists in a free form and is not associated with virus particles. The origin and biological functions of this secreted form of ORF2 (ORF2S) are unknown. Here we show that production of ORF2S results from translation initiated at the previously presumed AUG start codon for the capsid protein, whereas translation of the actual capsid protein (ORF2C) is initiated at a previously unrecognized internal AUG codon (15 codons downstream of the first AUG). The addition of 15 amino acids to the N terminus of the capsid protein creates a signal sequence that drives ORF2S secretion via the secretory pathway. Unlike ORF2C, ORF2S is glycosylated and exists as a dimer. Nonetheless, ORF2S exhibits substantial antigenic overlap with the capsid, but the epitopes predicted to bind the putative cell receptor are lost. Consistent with this, ORF2S does not block HEV cell entry but inhibits antibody-mediated neutralization. These results reveal a previously unrecognized aspect in HEV biology and shed new light on the immune evasion mechanisms and pathogenesis of this virus.
Keywords: antibody neutralization; hepatitis E virus; immunological decoy; leaky translation; quasi-envelopment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kamar N, et al. Hepatitis E virus infection. Nat Rev Dis Primers. 2017;3:17086. - PubMed
-
- Pérez-Gracia MT, Suay-García B, Mateos-Lindemann ML. Hepatitis E and pregnancy: Current state. Rev Med Virol. 2017;27:e1929. - PubMed
-
- Meng XJ. Zoonotic and foodborne transmission of hepatitis E virus. Semin Liver Dis. 2013;33:41–49. - PubMed
-
- Pavio N, Meng XJ, Doceul V. Zoonotic origin of hepatitis E. Curr Opin Virol. 2015;10:34–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

