CX3CR1 identifies PD-1 therapy-responsive CD8+ T cells that withstand chemotherapy during cancer chemoimmunotherapy
- PMID: 29669928
- PMCID: PMC5931117
- DOI: 10.1172/jci.insight.97828
CX3CR1 identifies PD-1 therapy-responsive CD8+ T cells that withstand chemotherapy during cancer chemoimmunotherapy
Abstract
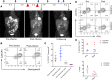
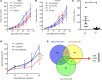
Although immune checkpoint inhibitors have resulted in durable clinical benefits in a subset of patients with advanced cancer, some patients who did not respond to initial anti-PD-1 therapy have been found to benefit from the addition of salvage chemotherapy. However, the mechanism responsible for the successful chemoimmunotherapy is not completely understood. Here we show that a subset of circulating CD8+ T cells expressing the chemokine receptor CX3CR1 are able to withstand the toxicity of chemotherapy and are increased in patients with metastatic melanoma who responded to chemoimmunotherapy (paclitaxel and carboplatin plus PD-1 blockade). These CX3CR1+CD8+ T cells have effector memory phenotypes and the ability to efflux chemotherapy drugs via the ABCB1 transporter. In line with clinical observation, our preclinical models identified an optimal sequencing of chemoimmunotherapy that resulted in an increase of CX3CR1+CD8+ T cells. Taken together, we found a subset of PD-1 therapy-responsive CD8+ T cells that were capable of withstanding chemotherapy and executing tumor rejection with their unique abilities of drug efflux (ABCB1), cytolytic activity (granzyme B and perforin), and migration to and retention (CX3CR1 and CD11a) at tumor sites. Future strategies to monitor and increase the frequency of CX3CR1+CD8+ T cells may help to design effective chemoimmunotherapy to overcome cancer resistance to immune checkpoint blockade therapy.
Keywords: Cancer immunotherapy; Cellular immune response; Immunology; Oncology; T cells.
Conflict of interest statement
Figures



References
-
- Yan Y, et al. The Mayo Clinic experience in patients with metastatic melanoma who have failed previous pembrolizumab treatment. J Clin Oncol. 2016;34((15_suppl);):e21014
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

