TSC2-deficient tumors have evidence of T cell exhaustion and respond to anti-PD-1/anti-CTLA-4 immunotherapy
- PMID: 29669930
- PMCID: PMC5931128
- DOI: 10.1172/jci.insight.98674
TSC2-deficient tumors have evidence of T cell exhaustion and respond to anti-PD-1/anti-CTLA-4 immunotherapy
Abstract
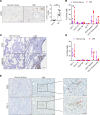
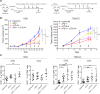
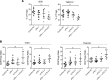
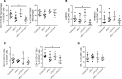
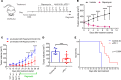
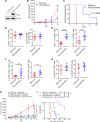
Tuberous sclerosis complex (TSC) is an incurable multisystem disease characterized by mTORC1-hyperactive tumors. TSC1/2 mutations also occur in other neoplastic disorders, including lymphangioleiomyomatosis (LAM) and bladder cancer. Whether TSC-associated tumors will respond to immunotherapy is unknown. We report here that the programmed death 1 coinhibitory receptor (PD-1) is upregulated on T cells in renal angiomyolipomas (AML) and pulmonary lymphangioleiomyomatosis (LAM). In C57BL/6J mice injected with syngeneic TSC2-deficient cells, anti-PD-1 alone decreased 105K tumor growth by 67% (P < 0.0001); the combination of PD-1 and CTLA-4 blockade was even more effective in suppressing tumor growth. Anti-PD-1 induced complete rejection of TSC2-deficient 105K tumors in 37% of mice (P < 0.05). Double blockade of PD-1 and CTLA-4 induced rejection in 62% of mice (P < 0.01). TSC2 reexpression in TSC2-deficient TMKOC cells enhanced antitumor immunity by increasing T cell infiltration and production of IFN-γ/TNF-α by T cells, suggesting that TSC2 and mTORC1 play specific roles in the induction of antitumor immunity. Finally, 1 month of anti-PD-1 blockade reduced renal tumor burden by 53% (P < 0.01) in genetically engineered Tsc2+/- mice. Taken together, these data demonstrate for the first time to our knowledge that checkpoint blockade may have clinical efficacy for TSC and LAM, and possibly other benign tumor syndromes, potentially yielding complete and durable clinical responses.
Keywords: Cancer immunotherapy; Immunotherapy; Oncology; T cells.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

