Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study
- PMID: 29669938
- PMCID: PMC5931122
- DOI: 10.1172/jci.insight.99123
Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study
Abstract
Background: Sodium glucose cotransporter-2 (SGLT2) inhibitors are the most recently approved class of drugs for type 2 diabetes and provide both glycemic efficacy and cardiovascular risk reduction. A number of safety issues have been identified, including treatment-emergent bone fractures. To understand the overall clinical profile, these safety issues must be balanced against an attractive efficacy profile. Our study was designed to investigate pathophysiological mechanisms mediating treatment-emergent adverse effects on bone health.
Methods: We conducted a single-blind randomized crossover study in hospitalized healthy adults (n = 25) receiving either canagliflozin (300 mg/d) or placebo for 5 days. The primary end-point was the drug-induced change in AUC for plasma intact fibroblast growth factor 23 (FGF23) immunoactivity between 24 and 72 hours.
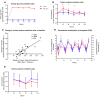
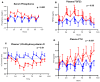
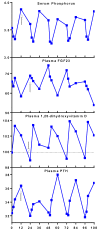
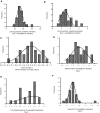
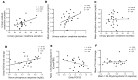
Results: Canagliflozin administration increased placebo-subtracted mean levels of serum phosphorus (+16%), plasma FGF23 (+20%), and plasma parathyroid hormone (PTH) (+25%), while decreasing the level of 1,25-dihydroxyvitamin D (-10%). There was substantial interindividual variation in the magnitude of each of these pharmacodynamic responses. The increase in plasma FGF23 was correlated with the increase in serum phosphorus, and the decrease in plasma 1,25-dihydroxyvitamin D was correlated with the increase in plasma FGF23.
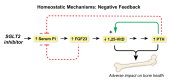
Conclusions: Canagliflozin induced a prompt increase in serum phosphorus, which triggers downstream changes in FGF23, 1,25-dihydroxyvitamin D, and PTH, with potential to exert adverse effects on bone health. These pharmacodynamic data provide a foundation for future research to elucidate pathophysiological mechanisms of adverse effects on bone health, with the objective of devising therapeutic strategies to mitigate the drug-associated fracture risk.
Trial registration: ClinicalTrial.gov (NCT02404870).
Funding: Supported by the Intramural Program of NIDDK.
Keywords: Bone Biology; Bone disease; Diabetes; Endocrinology.
Conflict of interest statement
Figures
References
-
- FDA Drug Safety Communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. Food and Drug Administration. http://www.fda.gov/Drugs/DrugSafety/ucm461449.htm Published September 10, 2015. Updated January 15, 2016. Accessed March 30, 2018.
-
- FDA Drug Safety Communication: Interim clinical trial results find increased risk of leg and foot amputations, mostly affecting the toes, with the diabetes medicine canagliflozin (Invokana, Invokamet); FDA to investigate. Food and Drug Administration. https://www.fda.gov/Drugs/DrugSafety/ucm500965.htm Published May 18, 2016. Updated May 17, 2017. Accessed March 30, 2018.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

