Cystic fibrosis-related diabetes is caused by islet loss and inflammation
- PMID: 29669939
- PMCID: PMC5931120
- DOI: 10.1172/jci.insight.98240
Cystic fibrosis-related diabetes is caused by islet loss and inflammation
Abstract
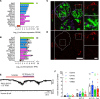
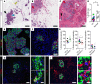
Cystic fibrosis-related (CF-related) diabetes (CFRD) is an increasingly common and devastating comorbidity of CF, affecting approximately 35% of adults with CF. However, the underlying causes of CFRD are unclear. Here, we examined cystic fibrosis transmembrane conductance regulator (CFTR) islet expression and whether the CFTR participates in islet endocrine cell function using murine models of β cell CFTR deletion and normal and CF human pancreas and islets. Specific deletion of CFTR from murine β cells did not affect β cell function. In human islets, CFTR mRNA was minimally expressed, and CFTR protein and electrical activity were not detected. Isolated CF/CFRD islets demonstrated appropriate insulin and glucagon secretion, with few changes in key islet-regulatory transcripts. Furthermore, approximately 65% of β cell area was lost in CF donors, compounded by pancreatic remodeling and immune infiltration of the islet. These results indicate that CFRD is caused by β cell loss and intraislet inflammation in the setting of a complex pleiotropic disease and not by intrinsic islet dysfunction from CFTR mutation.
Keywords: Cell Biology; Diabetes; Endocrinology; Genetic diseases; Islet cells.
Conflict of interest statement
Figures





References
-
- Cystic Fibrosis Foundation Patient Registry 2016 Annual Data Report. Cystic Fibrosis Foundation. https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2016-... Accessed April 10, 2018.
-
- Moran A, et al. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–2708. doi: 10.2337/dc10-1768. - DOI - PMC - PubMed
-
- Gudipaty L, Rickels MR. Pancreatogenic (Type 3c) Diabetes. The Pancreapedia: Exocrine Pancreas Knowledge Base. https://doi.org/10.3998/panc.2015.35.
-
- O’Riordan SM, Robinson PD, Donaghue KC, Moran A, ISPAD Clinical Practice Consensus Management of cystic fibrosis-related diabetes. Pediatr Diabetes. 2008;9(4 Pt 1):338–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DK007061/DK/NIDDK NIH HHS/United States
- UC4 DK104211/DK/NIDDK NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- R21 AI126189/AI/NIAID NIH HHS/United States
- R01 DK115620/DK/NIDDK NIH HHS/United States
- UC4 DK108120/DK/NIDDK NIH HHS/United States
- U01 DK072473/DK/NIDDK NIH HHS/United States
- UC4 DK112232/DK/NIDDK NIH HHS/United States
- U01 DK089572/DK/NIDDK NIH HHS/United States
- R24 DK106755/DK/NIDDK NIH HHS/United States
- R01 DK097829/DK/NIDDK NIH HHS/United States
- S10 OD021630/OD/NIH HHS/United States
- UC4 DK116284/DK/NIDDK NIH HHS/United States
- R01 DK094199/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

