Bone Cell Activity in Clinical Prostate Cancer Bone Metastasis and Its Inverse Relation to Tumor Cell Androgen Receptor Activity
- PMID: 29670000
- PMCID: PMC5979457
- DOI: 10.3390/ijms19041223
Bone Cell Activity in Clinical Prostate Cancer Bone Metastasis and Its Inverse Relation to Tumor Cell Androgen Receptor Activity
Abstract
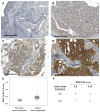
Advanced prostate cancer frequently metastasizes to bone and induces a mixed osteoblastic/osteolytic bone response. Standard treatment for metastatic prostate cancer is androgen-deprivation therapy (ADT) that also affects bone biology. Treatment options for patients relapsing after ADT are limited, particularly in cases where castration-resistance does not depend on androgen receptor (AR) activity. Patients with non-AR driven metastases may, however, benefit from therapies targeting the tumor microenvironment. Therefore, the current study specifically investigated bone cell activity in clinical bone metastases in relation to tumor cell AR activity, in order to gain novel insight into biological heterogeneities of possible importance for patient stratification into bone-targeting therapies. Metastasis tissue obtained from treatment-naïve (n = 11) and castration-resistant (n = 28) patients was characterized using whole-genome expression analysis followed by multivariate modeling, functional enrichment analysis, and histological evaluation. Bone cell activity was analyzed by measuring expression levels of predefined marker genes representing osteoclasts (ACP5, CTSK, MMP9), osteoblasts (ALPL, BGLAP, RUNX2) and osteocytes (SOST). Principal component analysis indicated a positive correlation between osteoblast and osteoclast activity and a high variability in bone cell activity between different metastases. Immunohistochemistry verified a positive correlation between runt-related transcription factor 2 (RUNX2) positive osteoblasts and tartrate-resistant acid phosphatase (TRAP, encoded by ACP5) positive osteoclasts lining the metastatic bone surface. No difference in bone cell activity was seen between treatment-naïve and castration-resistant patients. Importantly, bone cell activity was inversely correlated to tumor cell AR activity (measured as AR, FOXA1, HOXB13, KLK2, KLK3, NKX3-1, STEAP2, and TMPRSS2 expression) and to patient serum prostate-specific antigen (PSA) levels. Functional enrichment analysis indicated high bone morphogenetic protein (BMP) signaling in metastases with high bone cell activity and low tumor cell AR activity. This was confirmed by BMP4 immunoreactivity in tumor cells of metastases with ongoing bone formation, as determined by histological evaluation of van Gieson-stained sections. In conclusion, the inverse relation observed between bone cell activity and tumor cell AR activity in prostate cancer bone metastasis may be of importance for patient response to AR and/or bone targeting therapies, but needs to be evaluated in clinical settings in relation to serum markers for bone remodeling, radiography and patient response to therapy. The importance of BMP signaling in the development of sclerotic metastasis lesions deserves further exploration.
Keywords: BMP; androgen receptor; bone; metastasis; osteoblast; osteoclast; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- You S., Knudsen B.S., Erho N., Alshalalfa M., Takhar M., Ashab H.A.D., Davicioni E., Karnes R.J., Klein E.A., Den R.B., et al. Integrated Classification of Prostate Cancer Reveals a Novel Luminal Subtype with Poor Outcome. Cancer Res. 2016;76:4948–4958. doi: 10.1158/0008-5472.CAN-16-0902. - DOI - PMC - PubMed
-
- Zhao S.G., Chang S.L., Spratt D.E., Erho N., Yu M., Ashab H.A.D., Alshalalfa M., Speers C., Tomlins S.A., Davicioni E., et al. Development and Validation of a 24-Gene Predictor of Response to Postoperative Radiotherapy in Prostate Cancer: A Matched, Retrospective Analysis. Lancet Oncol. 2016;17:1612–1620. doi: 10.1016/S1470-2045(16)30491-0. - DOI - PubMed
-
- Hörnberg E., Ylitalo E.B., Crnalic S., Antti H., Stattin P., Widmark A., Bergh A., Wikström P. Expression of Androgen Receptor Splice Variants in Prostate Cancer Bone Metastases Is Associated with Castration-Resistance and Short Survival. PLoS ONE. 2011;6:e19059. doi: 10.1371/journal.pone.0019059. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

