Biophysical Investigations Elucidating the Mechanisms of Action of Antimicrobial Peptides and Their Synergism
- PMID: 29670065
- PMCID: PMC6023007
- DOI: 10.3390/biom8020018
Biophysical Investigations Elucidating the Mechanisms of Action of Antimicrobial Peptides and Their Synergism
Abstract
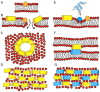
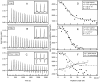
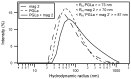
Biophysical and structural investigations are presented with a focus on the membrane lipid interactions of cationic linear antibiotic peptides such as magainin, PGLa, LL37, and melittin. Observations made with these peptides are distinct as seen from data obtained with the hydrophobic peptide alamethicin. The cationic amphipathic peptides predominantly adopt membrane alignments parallel to the bilayer surface; thus the distribution of polar and non-polar side chains of the amphipathic helices mirror the environmental changes at the membrane interface. Such a membrane partitioning of an amphipathic helix has been shown to cause considerable disruptions in the lipid packing arrangements, transient openings at low peptide concentration, and membrane disintegration at higher peptide-to-lipid ratios. The manifold supramolecular arrangements adopted by lipids and peptides are represented by the 'soft membranes adapt and respond, also transiently' (SMART) model. Whereas molecular dynamics simulations provide atomistic views on lipid membranes in the presence of antimicrobial peptides, the biophysical investigations reveal interesting details on a molecular and supramolecular level, and recent microscopic imaging experiments delineate interesting sequences of events when bacterial cells are exposed to such peptides. Finally, biophysical studies that aim to reveal the mechanisms of synergistic interactions of magainin 2 and PGLa are presented, including unpublished isothermal titration calorimetry (ITC), circular dichroism (CD) and dynamic light scattering (DLS) measurements that suggest that the peptides are involved in liposome agglutination by mediating intermembrane interactions. A number of structural events are presented in schematic models that relate to the antimicrobial and synergistic mechanism of amphipathic peptides when they are aligned parallel to the membrane surface.
Keywords: carpet model; cecropin; local disorder; magainin; membrane macroscopic phase; membrane pore; membrane topology; peptide-lipid interactions; soft membrane adapt and respond, also transiently (SMART) model; toroidal pore.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

