Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis
- PMID: 29670287
- PMCID: PMC6037913
- DOI: 10.1038/s41586-018-0052-z
Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis
Abstract
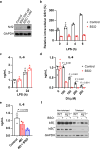
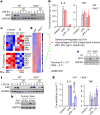
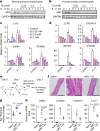
Metabolic regulation has been recognized as a powerful principle guiding immune responses. Inflammatory macrophages undergo extensive metabolic rewiring 1 marked by the production of substantial amounts of itaconate, which has recently been described as an immunoregulatory metabolite 2 . Itaconate and its membrane-permeable derivative dimethyl itaconate (DI) selectively inhibit a subset of cytokines 2 , including IL-6 and IL-12 but not TNF. The major effects of itaconate on cellular metabolism during macrophage activation have been attributed to the inhibition of succinate dehydrogenase2,3, yet this inhibition alone is not sufficient to account for the pronounced immunoregulatory effects observed in the case of DI. Furthermore, the regulatory pathway responsible for such selective effects of itaconate and DI on the inflammatory program has not been defined. Here we show that itaconate and DI induce electrophilic stress, react with glutathione and subsequently induce both Nrf2 (also known as NFE2L2)-dependent and -independent responses. We find that electrophilic stress can selectively regulate secondary, but not primary, transcriptional responses to toll-like receptor stimulation via inhibition of IκBζ protein induction. The regulation of IκBζ is independent of Nrf2, and we identify ATF3 as its key mediator. The inhibitory effect is conserved across species and cell types, and the in vivo administration of DI can ameliorate IL-17-IκBζ-driven skin pathology in a mouse model of psoriasis, highlighting the therapeutic potential of this regulatory pathway. Our results demonstrate that targeting the DI-IκBζ regulatory axis could be an important new strategy for the treatment of IL-17-IκBζ-mediated autoimmune diseases.
Conflict of interest statement
Figures








Comment in
-
Itaconate charges down inflammation.Nat Rev Immunol. 2018 Jun;18(6):360-361. doi: 10.1038/s41577-018-0016-4. Nat Rev Immunol. 2018. PMID: 29725119 No abstract available.
References
-
- Jha AK, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. - PubMed
-
- Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12:931–947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

