Relapsing Fevers: Neglected Tick-Borne Diseases
- PMID: 29670860
- PMCID: PMC5893795
- DOI: 10.3389/fcimb.2018.00098
Relapsing Fevers: Neglected Tick-Borne Diseases
Abstract
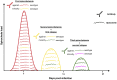
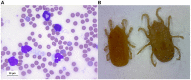
Relapsing fever still remains a neglected disease and little is known on its reservoir, tick vector and physiopathology in the vertebrate host. The disease occurs in temperate as well as tropical countries. Relapsing fever borreliae are spirochaetes, members of the Borreliaceae family which also contain Lyme disease spirochaetes. They are mainly transmitted by Ornithodoros soft ticks, but some species are vectored by ixodid ticks. Traditionally a Borrelia species is associated with a specific vector in a particular geographical area. However, new species are regularly described, and taxonomical uncertainties deserve further investigations to better understand Borrelia vector/host adaptation. The medical importance of Borrelia miyamotoi, transmitted by Ixodes spp., has recently spawned new interest in this bacterial group. In this review, recent data on tick-host-pathogen interactions for tick-borne relapsing fevers is presented, with special focus on B. miyamotoi.
Keywords: Borrelia; Borrelia miyamotoi; Ornithodoros; antigenic variations; hard ticks; neurotropism; relapsing fever.
Figures


References
-
- Adeolu M., Gupta R. S. (2014). A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: the emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Van Leeuwenhoek 105, 1049–1072. 10.1007/s10482-014-0164-x - DOI - PubMed
-
- Ahmed M. A., Abdel Wahab S. M., Abdel Malik M. O., Abdel Gadir A. M., Salih S. Y., Omer A., et al. (1980). Louse-borne relapsing fever in the Sudan. A historical review and a clinico-pathological study. Trop. Geogr. Med. 32, 106–111. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

