Cathelicidin LL-37 Affects Surface and Intracellular Toll-Like Receptor Expression in Tissue Mast Cells
- PMID: 29670923
- PMCID: PMC5836302
- DOI: 10.1155/2018/7357162
Cathelicidin LL-37 Affects Surface and Intracellular Toll-Like Receptor Expression in Tissue Mast Cells
Abstract
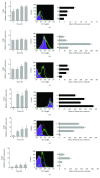
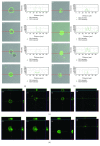
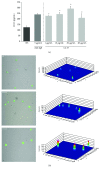
Undoubtedly, mast cells take part in host defense against microorganisms as they are numerous at the portal of infection, they release many proinflammatory and antimicrobial mediators, and they express pattern recognition receptors, such as TLRs. These receptors play a key role in recognition and binding molecules associated with microorganisms and molecules associated with damage. Cathelicidins exhibit direct antimicrobial activities against a broad spectrum of microbes by perturbing their cell membranes. Accumulating evidence suggests a role for these molecules in supporting cell activation. We examined the impact of human cathelicidin LL-37 on tissue mast cell TLR expression and distribution. Depending on context, we show that LL-37 stimulation resulted in minor to major effects on TLR2, TLR3, TLR4, TLR5, TLR7, and TLR9 expression. Confocal microscopy analysis showed that, upon stimulation, TLRs may translocate from the cell interior to the surface and conversely. FPR2 and EGFR inhibitors reduced the increase in expression of selected receptors. We also established that LL-37 acts as a powerful inducer of CCL3 and ROS generation. These results showed that in response to LL-37, mast cells enhance the capability to detect invading pathogens by modulation of TLR expression in what may be involved FPR2 or EGFR molecules.
Figures






Similar articles
-
Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells.Exp Eye Res. 2011 Mar;92(3):209-20. doi: 10.1016/j.exer.2010.12.005. Epub 2010 Dec 31. Exp Eye Res. 2011. PMID: 21195713 Free PMC article.
-
LL-37-induced human mast cell activation through G protein-coupled receptor MrgX2.Int Immunopharmacol. 2017 Aug;49:6-12. doi: 10.1016/j.intimp.2017.05.016. Epub 2017 May 23. Int Immunopharmacol. 2017. PMID: 28549244
-
[Mast cell Toll-like receptors (TLRs)].Postepy Hig Med Dosw (Online). 2010 Jan 20;64:11-21. Postepy Hig Med Dosw (Online). 2010. PMID: 20093719 Review. Polish.
-
An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7.J Immunol. 2006 Mar 1;176(5):3044-52. doi: 10.4049/jimmunol.176.5.3044. J Immunol. 2006. PMID: 16493063
-
Tissue-specific Regulation of Innate Immune Responses by Human Cathelicidin LL-37.Curr Pharm Des. 2018;24(10):1079-1091. doi: 10.2174/1381612824666180327113418. Curr Pharm Des. 2018. PMID: 29589544 Review.
Cited by
-
Cathelicidin Host Defense Peptides and Inflammatory Signaling: Striking a Balance.Front Microbiol. 2020 Aug 27;11:1902. doi: 10.3389/fmicb.2020.01902. eCollection 2020. Front Microbiol. 2020. PMID: 32982998 Free PMC article. Review.
-
Significance of Mast Cell Formed Extracellular Traps in Microbial Defense.Clin Rev Allergy Immunol. 2022 Feb;62(1):160-179. doi: 10.1007/s12016-021-08861-6. Epub 2021 May 22. Clin Rev Allergy Immunol. 2022. PMID: 34024033 Free PMC article. Review.
-
A novel definition and treatment of hyperinflammation in COVID-19 based on purinergic signalling.Purinergic Signal. 2022 Mar;18(1):13-59. doi: 10.1007/s11302-021-09814-6. Epub 2021 Nov 10. Purinergic Signal. 2022. PMID: 34757513 Free PMC article. Review.
-
KR‑12‑a6 promotes the osteogenic differentiation of human bone marrow mesenchymal stem cells via BMP/SMAD signaling.Mol Med Rep. 2020 Jan;21(1):61-68. doi: 10.3892/mmr.2019.10843. Epub 2019 Nov 22. Mol Med Rep. 2020. PMID: 31939626 Free PMC article.
-
Signal Transduction Pathways Activated by Innate Immunity in Mast Cells: Translating Sensing of Changes into Specific Responses.Cells. 2020 Nov 4;9(11):2411. doi: 10.3390/cells9112411. Cells. 2020. PMID: 33158024 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

