Mechanisms involved in normal and pathological osteoclastogenesis
- PMID: 29670999
- PMCID: PMC9809143
- DOI: 10.1007/s00018-018-2817-9
Mechanisms involved in normal and pathological osteoclastogenesis
Abstract
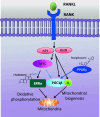
Osteoclasts are bone-resorbing cells that play an essential role in bone remodeling. Defects in osteoclasts result in unbalanced bone remodeling and are linked to many bone diseases including osteoporosis, rheumatoid arthritis, primary bone cancer, and skeletal metastases. Receptor activator of NF-kappaB ligand (RANKL) is a classical inducer of osteoclast formation. In the presence of macrophage-colony-stimulating factor, RANKL and co-stimulatory signals synergistically regulate osteoclastogenesis. However, recent discoveries of alternative pathways for RANKL-independent osteoclastogenesis have led to a reassessment of the traditional mechanisms that regulate osteoclast formation. In this review, we provide an overview of signaling pathways and other regulatory elements governing osteoclastogenesis. We also identify how osteoclastogenesis is altered in pathological conditions and discuss therapeutic targets in osteoclasts for the treatment of skeletal diseases.
Keywords: MYC; Osteoclastogenesis; Osteoclasts; RANK; RANKL.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

