Role of Ca2+ in changing active force during intermittent submaximal stimulation in intact, single mouse muscle fibers
- PMID: 29671103
- PMCID: PMC6060763
- DOI: 10.1007/s00424-018-2143-y
Role of Ca2+ in changing active force during intermittent submaximal stimulation in intact, single mouse muscle fibers
Abstract
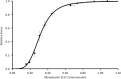
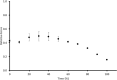
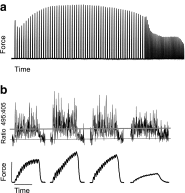
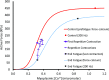
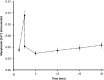
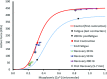
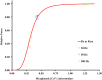
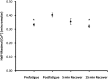
Fatigue of single mouse fibers during repeated high-frequency stimulation results initially from decreased Ca2+ sensitivity while free myoplasmic calcium concentration ([Ca2+]m) increases, followed by decreasing [Ca2+]m. Recovery of active force with low-frequency stimulation is slow and persistent fatigue results from low [Ca2+]m. However, the consequences of intermittent submaximal contractions are not known. The aim of the present study was to investigate the changes in [Ca2+]m and active force during intermittent submaximal contractions and subsequent recovery. Single fibers of mouse flexor digitorum brevis muscles at 32 °C were stimulated with 40 or 50 Hz, for 350 ms every 2 s for 2 min and then every 1 s until < 40% of initial force. Values obtained during the intermittent stimulation were compared with a control force-[Ca2+]m relationship. A "P"-shaped pattern in the force-[Ca2+]m relationship was observed during intermittent stimulation. Early in the intermittent stimulation, [Ca2+]m increased while active force decreased. Subsequent force potentiation was accompanied by increased Ca2+ sensitivity. Later, as active force declined, [Ca2+]m decreased significantly (p < 0.001). This was followed, in the final phase, by a significant decrease in Ca2+ sensitivity determined by [Ca2+]m at half-maximal force (Ca50) (p = 0.001). Low-frequency fatigue persisted during recovery while Ca50 was not significantly different from prefatigue (p > 0.5). In conclusion, the main mechanism of fatigue is due to decreases in both [Ca2+]m and Ca2+ sensitivity following the initial force potentiation. The intermittent submaximal contractions resulted in persistent low-frequency fatigue seen during recovery, which was explained by depressed [Ca2+]m with no change in Ca2+ sensitivity.
Keywords: Calcium sensitivity; Fatigue; Half-maximal calcium concentration; Potentiation; Staircase.
Conflict of interest statement
Ethical approval
All procedures were approved by the University of Calgary Animal Care Committee and by the Stockholm North Ethical Committee on Animal Experiments at Karolinska Institutet.
Competing interests
None.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

