Human Articular Chondrocytes Retain Their Phenotype in Sustained Hypoxia While Normoxia Promotes Their Immunomodulatory Potential
- PMID: 29671342
- PMCID: PMC6755872
- DOI: 10.1177/1947603518769714
Human Articular Chondrocytes Retain Their Phenotype in Sustained Hypoxia While Normoxia Promotes Their Immunomodulatory Potential
Abstract
Objective: To assess the phenotype of human articular chondrocytes cultured in normoxia (21% O2) or continuous hypoxia (2% O2).
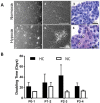
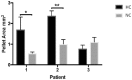
Design: Chondrocytes were extracted from patients undergoing total knee replacement (n = 5) and cultured in ~21% (normoxic chondrocytes, NC) and 2% (hypoxic chondrocytes, HC) oxygen in both monolayer and 3-dimensional (3D) pellet culture and compared with freshly isolated chondrocytes (FC). Cells were assessed by flow cytometry for markers indicative of mesenchymal stromal cells (MSCs), chondrogenic-potency and dedifferentiation. Chondrogenic potency and immunomodulatory gene expression was assessed in NC and HC by reverse transcription quantitative polymerase chain reaction. Immunohistochemistry was used to assess collagen II production following 3D pellet culture.
Results: NC were positive (>97%, n = 5) for MSC markers, CD73, CD90, and CD105, while HC demonstrated <90% positivity (n = 4) and FC (n = 5) less again (CD73 and CD90 <20%; CD105 <40%). The markers CD166 and CD151, indicative of chondrogenic de-differentiation, were significantly higher on NC compared with HC and lowest on FC. NC also produced the highest levels of CD106 and showed the greatest levels of IDO expression, following interferon-γ stimulation, indicating immunomodulatory potential. NC produced the highest levels of CD49c (>60%) compared with HC and FC in which production was <2%. Hypoxic conditions upregulated expression of SOX9, frizzled-related protein (FRZB), fibroblast growth factor receptor 3 (FGFR3), and collagen type II (COL2A1) and downregulated activin receptor-like kinase 1 (ALK1) in 3 out of 4 patients compared with normoxic conditions for monolayer cells.
Conclusions: Hypoxic conditions encourage retention of a chondrogenic phenotype with some immunomodulatory potential, whereas normoxia promotes dedifferentiation of chondrocytes toward an MSC phenotype with loss of chondrogenic potency but enhanced immunomodulatory capacity.
Keywords: Sustained hypoxia; cartilage repair; chondrogenic; hypoxic workstation; immunomodulation.
Conflict of interest statement
Figures






References
-
- Silver IA. Measurement of pH and ionic composition of pericellular sites. Philos Trans R Soc Lond B Biol Sci. 1975;271(912):261-72. - PubMed
-
- Duval E, Leclercq S, Elissalde JM, Demoor M, Galéra P, Boumédiene K. Hypoxia-inducible factor 1α inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation: hypoxia not only induces type II collagen and aggrecan, but it also inhibits type I and type III collagen in the hypoxia-inducible factor 1α-dependent redifferentiation of chondrocytes. Arthritis Rheum. 2009;60(10):3038-48. doi:10.1002/art.24851. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

