Optochemical Control of Protein Localization and Activity within Cell-like Compartments
- PMID: 29671583
- PMCID: PMC7330991
- DOI: 10.1021/acs.biochem.8b00131
Optochemical Control of Protein Localization and Activity within Cell-like Compartments
Abstract
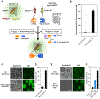
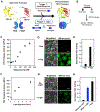
We report inducible dimerization strategies for controlling protein positioning, enzymatic activity, and organelle assembly inside synthetic cell-like compartments upon photostimulation. Using a photocaged TMP-Haloligand compound, we demonstrate small molecule and light-induced dimerization of DHFR and Haloenzyme to localize proteins to a compartment boundary and reconstitute tripartite sfGFP assembly. Using photocaged rapamycin and fragments of split TEV protease fused to FRB and FKBP, we establish optical triggering of protease activity inside cell-size compartments. We apply light-inducible protease activation to initiate assembly of membraneless organelles, demonstrating the applicability of these tools for characterizing cell biological processes in vitro. This modular toolkit, which affords spatial and temporal control of protein function in a minimal cell-like system, represents a critical step toward the reconstitution of a tunable synthetic cell, built from the bottom up.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

