Improving the Activity of Trp-Rich Antimicrobial Peptides by Arg/Lys Substitutions and Changing the Length of Cationic Residues
- PMID: 29671805
- PMCID: PMC6023086
- DOI: 10.3390/biom8020019
Improving the Activity of Trp-Rich Antimicrobial Peptides by Arg/Lys Substitutions and Changing the Length of Cationic Residues
Abstract
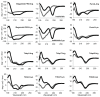
Antimicrobial peptides (AMPs) constitute a promising alternative for the development of new antibiotics that could potentially counteract the growing number of antibiotic-resistant bacteria. However, the AMP structure⁻function relationships remain unclear and detailed studies are still necessary. The positively charged amino acid residues (Arg and Lys) play a crucial role in the activity of most AMPs due to the promotion of electrostatic interactions between the peptides and bacterial membranes. In this work we have analyzed the antimicrobial and structural properties of several Trp-rich AMPs containing exclusively either Arg or Lys as the positively charged residues. Their antimicrobial activity and mechanism of action were investigated, showing that Lys residues give rise to a reduced antimicrobial potency for most peptides, which was correlated, in turn, with a decrease in their ability to permeabilize the cytoplasmic membrane of Escherichia coli. Additionally, the presence of Arg and Lys renders the peptides susceptible to degradation by proteases, such as trypsin, limiting their therapeutic use. Therefore, modifications of the side chain length of Arg and Lys were investigated in an attempt to improve the protease resistance of AMPs. This approach resulted in enhanced stability to trypsin digestion, and in several cases, shorter sidechains conserved or even improved the antimicrobial activity. All together, these results suggest that Arg-to-Lys substitutions, coupled with side chain length modifications, can be extremely useful for improving the activity and stability of AMPs.
Keywords: antimicrobial peptides; arginine; lysine; protease degradation; tritrpticin; trypsin.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript, nor in the decision to publish the results.
Figures




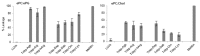


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

