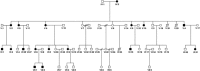
Effects of SYN1Q555X mutation on cortical gray matter microstructure
- PMID: 29671924
- PMCID: PMC6866302
- DOI: 10.1002/hbm.24186
Effects of SYN1Q555X mutation on cortical gray matter microstructure
Abstract
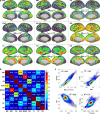
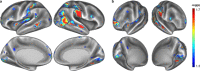
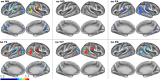
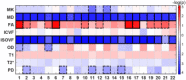
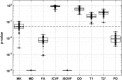
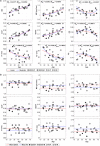
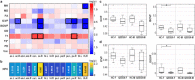
A new Q555X mutation on the SYN1 gene was recently found in several members of a family segregating dyslexia, epilepsy, and autism spectrum disorder. To describe the effects of this mutation on cortical gray matter microstructure, we performed a surface-based group study using novel diffusion and quantitative multiparametric imaging on 13 SYN1Q555X mutation carriers and 13 age- and sex-matched controls. Specifically, diffusion kurtosis imaging (DKI) and neurite orientation and dispersion and density imaging (NODDI) were used to analyze multi-shell diffusion data and obtain parametric maps sensitive to tissue structure, while quantitative metrics sensitive to tissue composition (T1, T2* and relative proton density [PD]) were obtained from a multi-echo variable flip angle FLASH acquisition. Results showed significant microstructural alterations in several regions usually involved in oral and written language as well as dyslexia. The most significant changes in these regions were lowered mean diffusivity and increased fractional anisotropy. This study is, to our knowledge, the first to successfully use diffusion imaging and multiparametric mapping to detect cortical anomalies in a group of subjects with a well-defined genotype linked to language impairments, epilepsy and autism spectrum disorder (ASD).
Keywords: MRI; adult; autism; cortex; dyslexia; epilepsy; genetics.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Guillaume Gilbert receives salary from Philips Healthcare for work outside of the scope of this study.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

