Sex differences in opioid use and medical issues during buprenorphine/naloxone treatment
- PMID: 29672167
- PMCID: PMC6186400
- DOI: 10.1080/00952990.2018.1458234
Sex differences in opioid use and medical issues during buprenorphine/naloxone treatment
Abstract
Background: There are sex differences in buprenorphine/naloxone clinical trials for opioid use. While women have fewer opioid-positive urine samples, relative to men, a significant decrease in opioid-positive samples was found during treatment for men, but not women. In order to inform sex-based approaches to improve treatment outcomes, research is needed to determine if opioid use, and predictors of opioid use, differs between men and women during treatment.
Objectives: To test for sex differences in opioid use during a buprenorphine/naloxone clinical trial and determine if sex differences exist in the associations between addiction-related problem areas and opioid use over the course of the trial.
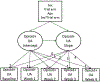
Method: This secondary data analysis of the National Drug Abuse Treatment Clinical Trials Network (CTN) 0003 examined sex differences (men = 347, women = 169) in opioid-positive samples in a randomized clinical trial comparing 7-day vs. 28-day buprenorphine/naloxone tapering strategies. Addiction-related problem areas were defined by Addiction Severity-Lite (ASI-L) domain composite scores.
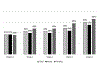
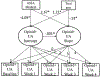
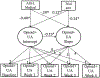
Results: Women were more likely than men to use opioids during the course of the buprenorphine/naloxone clinical trial (B = .33, p = .01) and medical issues were positively related to submitting an opioid-positive sample during treatment for women (B = 1.67, p = .01). No ASI-L domain composite score was associated with opioid-positive samples during treatment for men.
Conclusion: Women were more likely than men to use opioids during the course of the buprenorphine/naloxone clinical trial, and medical issues predicted opioid use during treatment for women but not men. Complementary treatment for medical problems during opioid replacement therapy may benefit women.
Keywords: Opioid dependence; addiction severity index; buprenorphine/naloxone; sex differences.
Figures
References
-
- Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health (HHS Publication No. SMA 16–4984, NSDUH Series H-51). 2016.
-
- Jones HE, Fitzgerald H, Johnson RE. Males and females differ in response to opioid agonist medications. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2005;14(3):223–33. Epub 2005/07/16. - PubMed
-
- CDC. Vital Signs: Prescription Painkiller Overdoses. A Growing Epidemic, Especially Among Women. July 2013; Available from: http://www.cdc.gov/vitalsigns/prescriptionpainkilleroverdoses/index.html.
-
- Kaiser Family Foundation. Opioid Overdose Deaths by Gender. 2017; Available from: http://www.kff.org/other/state-indicator/opioid-overdose-deaths-by-gende....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
