Macrophage-derived extracellular vesicles mediate smooth muscle hyperplasia: role of altered miRNA cargo in response to HIV infection and substance abuse
- PMID: 29672222
- PMCID: PMC6103174
- DOI: 10.1096/fj.201701558R
Macrophage-derived extracellular vesicles mediate smooth muscle hyperplasia: role of altered miRNA cargo in response to HIV infection and substance abuse
Erratum in
-
corrigendum.FASEB J. 2021 Nov;35(11):e22020. doi: 10.1096/fsb2.22020. FASEB J. 2021. PMID: 34674315 Free PMC article. No abstract available.
Abstract
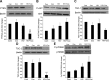
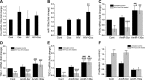
Our previous studies consistently demonstrate enhanced pulmonary vascular remodeling in HIV-infected intravenous drug users, and in simian immunodeficiency virus-infected macaques or HIV-transgenic rats exposed to opioids or cocaine. Although we reported an associated increase in perivascular inflammation, the exact role of inflammatory cells in the development of pulmonary vascular remodeling remains unknown. In this study, HIV-infected and cocaine (H+C)-treated human monocyte derived macrophages released a higher number of extracellular vesicles (EVs), compared to HIV-infected or uninfected cocaine-treated macrophages, with a significant increase in the particle size range to 100-150 nm. Treatment of primary human pulmonary arterial smooth muscle cells (HPASMCs) with these EVs resulted in a significant increase in smooth muscle proliferation. We also observed a significant increase in the miRNA-130a level in the EVs derived from H+C-treated macrophages that corresponded with the decrease in the expression of phosphatase and tensin homolog and tuberous sclerosis 1 and 2 and activation of PI3K/protein kinase B signaling in HPASMCs on addition of these EVs. Transfection of HPASMCs with antagomir-130a-ameliorated the EV-induced effect. Thus, we conclude that EVs derived from H+C-treated macrophages promote pulmonary smooth muscle proliferation by delivery of its prosurvival miRNA cargo, which may play a crucial role in the development of PAH.-Sharma, H., Chinnappan, M., Agarwal, S., Dalvi, P., Gunewardena, S., O'Brien-Ladner, A., Dhillon, N. K. Macrophage-derived extracellular vesicles mediate smooth muscle hyperplasia: role of altered miRNA cargo in response to HIV infection and substance abuse.
Keywords: HIV-PAH; cocaine; miR-130a; pulmonary vascular remodelling.
Conflict of interest statement
The authors thank the Electron Microscopy Research Laboratory (EMRL) facility and Barbara Fegley for assistance with electron microscopy of isolate extracellular vesicles; Dr. Lane Christenson for providing access to NanoSight-LM10; the Kansas Intellectual and Developmental Disabilities Research Center, U. S. National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant U54 HD 090216; and the Molecular Regulation of Cell Development and Differentiation Centers of Biomedical Research Excellence (COBRE) Grant 5P20GM104936-10 (University of Kansas Medical Center for RNA Sequencing and Bioinformatics Services). This work was supported by NIH National Institute on Drug Abuse Grants R01DA034542 and R01DA042715, and NIH National Heart, Blood, and Lung Institute R01HL129875 (to N.K.D.). The authors declare no conflicts of interest.
Figures




References
-
- Sitbon O., Lascoux-Combe C., Delfraissy J. F., Yeni P. G., Raffi F., De Zuttere D., Gressin V., Clerson P., Sereni D., Simonneau G. (2008) Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am. J. Respir. Crit. Care Med. 177, 108–113 - PubMed
-
- Humbert M., Sitbon O., Chaouat A., Bertocchi M., Habib G., Gressin V., Yaici A., Weitzenblum E., Cordier J. F., Chabot F., Dromer C., Pison C., Reynaud-Gaubert M., Haloun A., Laurent M., Hachulla E., Simonneau G. (2006) Pulmonary arterial hypertension in France: results from a national registry. Am. J. Respir. Crit. Care Med. 173, 1023–1030 - PubMed
-
- Reinsch N., Buhr C., Krings P., Kaelsch H., Kahlert P., Konorza T., Neumann T., Erbel R.; Competence Network of Heart Failure (2008) Effect of gender and highly active antiretroviral therapy on HIV-related pulmonary arterial hypertension: results of the HIV-HEART study. HIV Med. 9, 550–556 - PubMed
-
- Morris A., Gingo M. R., George M. P., Lucht L., Kessinger C., Singh V., Hillenbrand M., Busch M., McMahon D., Norris K. A., Champion H. C., Gladwin M. T., Zhang Y., Steele C., Sciurba F. C. (2012) Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS 26, 731–740 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

