Use of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: Divergent policies and practices in malaria endemic countries
- PMID: 29672516
- PMCID: PMC5908060
- DOI: 10.1371/journal.pntd.0006230
Use of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: Divergent policies and practices in malaria endemic countries
Abstract
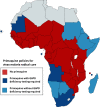
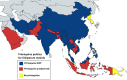
Primaquine is the only available antimalarial drug that kills dormant liver stages of Plasmodium vivax and Plasmodium ovale malarias and therefore prevents their relapse ('radical cure'). It is also the only generally available antimalarial that rapidly sterilises mature P. falciparum gametocytes. Radical cure requires extended courses of primaquine (usually 14 days; total dose 3.5-7 mg/kg), whereas transmissibility reduction in falciparum malaria requires a single dose (formerly 0.75 mg/kg, now a single low dose [SLD] of 0.25 mg/kg is recommended). The main adverse effect of primaquine is dose-dependent haemolysis in glucose 6-phosphate dehydrogenase (G6PD) deficiency, the most common human enzymopathy. X-linked mutations conferring varying degrees of G6PD deficiency are prevalent throughout malaria-endemic regions. Phenotypic screening tests usually detect <30% of normal G6PD activity, identifying nearly all male hemizygotes and female homozygotes and some heterozygotes. Unfortunately, G6PD deficiency screening is usually unavailable at point of care, and, as a consequence, radical cure is greatly underused. Both haemolytic risk (determined by the prevalence and severity of G6PD deficiency polymorphisms) and relapse rates vary, so there has been considerable uncertainty in both policies and practices related to G6PD deficiency testing and use of primaquine for radical cure. Review of available information on the prevalence and severity of G6PD variants together with countries' policies for the use of primaquine and G6PD deficiency testing confirms a wide range of practices. There remains lack of consensus on the requirement for G6PD deficiency testing before prescribing primaquine radical cure regimens. Despite substantially lower haemolytic risks, implementation of SLD primaquine as a P. falciparum gametocytocide also varies. In Africa, a few countries have recently adopted SLD primaquine, yet many with areas of low seasonal transmission do not use primaquine as an antimalarial at all. Most countries that recommended the higher 0.75 mg/kg single primaquine dose for falciparum malaria (e.g., most countries in the Americas) have not changed their recommendation. Some vivax malaria-endemic countries where G6PD deficiency testing is generally unavailable have adopted the once-weekly radical cure regimen (0.75 mg/kg/week for 8 weeks), known to be safer in less severe G6PD deficiency variants. There is substantial room for improvement in radical cure policies and practices.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: NJW was co-chairman, and JR and EAA were members of the World Health Organization Global Malaria Programme Primaquine Evidence Review group in 2012, although this review was initiated later and reflects the opinions of the authors and not their associated organisations.
Figures




References
-
- Beutler E, Duparc S (2007) Glucose-6-phosphate dehydrogenase deficiency and antimalarial drug development. Am J Trop Med Hyg 77(4): 779–789. - PubMed
-
- Cappellini MD, Fiorelli G (2008) Glucose-6-phosphate dehydrogenase deficiency. Lancet 371(9606): 64–74. doi: 10.1016/S0140-6736(08)60073-2 - DOI - PubMed
-
- Luzzatto L, Poggi V (2009) Glucose-6-Phosphate Dehydrogenase Deficiency (chapter 17) In: Orkin S, editor. Nathan and Oski's hematology of infancy and childhood, 7th edition Elsevier, Saunders, Philadelphia PA.
-
- Luzzatto L, Seneca E (2014) G6PD deficiency: a classic example of pharmacogenetics with on-going clinical implications. Br J Haematol 164(4): 469–480. doi: 10.1111/bjh.12665 - DOI - PMC - PubMed
-
- Luzzatto L, Nannelli C, Notaro R (2016) Glucose-6-Phosphate Dehydrogenase Deficiency. Hematol Oncol Clin North Am 30(2): 373–393. doi: 10.1016/j.hoc.2015.11.006 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

