EmbryoMiner: A new framework for interactive knowledge discovery in large-scale cell tracking data of developing embryos
- PMID: 29672531
- PMCID: PMC5929571
- DOI: 10.1371/journal.pcbi.1006128
EmbryoMiner: A new framework for interactive knowledge discovery in large-scale cell tracking data of developing embryos
Abstract
State-of-the-art light-sheet and confocal microscopes allow recording of entire embryos in 3D and over time (3D+t) for many hours. Fluorescently labeled structures can be segmented and tracked automatically in these terabyte-scale 3D+t images, resulting in thousands of cell migration trajectories that provide detailed insights to large-scale tissue reorganization at the cellular level. Here we present EmbryoMiner, a new interactive open-source framework suitable for in-depth analyses and comparisons of entire embryos, including an extensive set of trajectory features. Starting at the whole-embryo level, the framework can be used to iteratively focus on a region of interest within the embryo, to investigate and test specific trajectory-based hypotheses and to extract quantitative features from the isolated trajectories. Thus, the new framework provides a valuable new way to quantitatively compare corresponding anatomical regions in different embryos that were manually selected based on biological prior knowledge. As a proof of concept, we analyzed 3D+t light-sheet microscopy images of zebrafish embryos, showcasing potential user applications that can be performed using the new framework.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
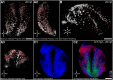

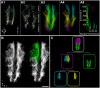


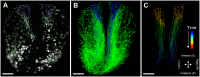
References
-
- Lecuit T, Le Goff L. Orchestrating Size and Shape during Morphogenesis. Nature. 2007;450(7167):189–192. doi: 10.1038/nature06304 - DOI - PubMed
-
- Giurumescu CA, Kang S, Planchon TA, Betzig E, Bloomekatz J, Yelon D, et al. Quantitative Semi-automated Analysis of Morphogenesis with Single-cell Resolution in Complex Embryos. Development. 2012;139(22):4271–4279. doi: 10.1242/dev.086256 - DOI - PMC - PubMed
-
- Keller PJ. Imaging Morphogenesis: Technological Advances and Biological Insights. Science. 2013;340(6137):1234168 doi: 10.1126/science.1234168 - DOI - PubMed
-
- Amat F, Lemon W, Mossing DP, McDole K, Wan Y, Branson K, et al. Fast, Accurate Reconstruction of Cell Lineages from Large-Scale Fluorescence Microscopy Data. Nature Methods. 2014;11(9):951–958. doi: 10.1038/nmeth.3036 - DOI - PubMed
-
- Fangerau J. Interactive Similarity Analysis for 3D+ t Cell Trajectory Data. Heidelberg University; 2015.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

