Infant transmitted/founder HIV-1 viruses from peripartum transmission are neutralization resistant to paired maternal plasma
- PMID: 29672607
- PMCID: PMC5908066
- DOI: 10.1371/journal.ppat.1006944
Infant transmitted/founder HIV-1 viruses from peripartum transmission are neutralization resistant to paired maternal plasma
Abstract
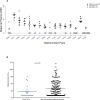
Despite extensive genetic diversity of HIV-1 in chronic infection, a single or few maternal virus variants become the founders of an infant's infection. These transmitted/founder (T/F) variants are of particular interest, as a maternal or infant HIV vaccine should raise envelope (Env) specific IgG responses capable of blocking this group of viruses. However, the maternal or infant factors that contribute to selection of infant T/F viruses are not well understood. In this study, we amplified HIV-1 env genes by single genome amplification from 16 mother-infant transmitting pairs from the U.S. pre-antiretroviral era Women Infant Transmission Study (WITS). Infant T/F and representative maternal non-transmitted Env variants from plasma were identified and used to generate pseudoviruses for paired maternal plasma neutralization sensitivity analysis. Eighteen out of 21 (85%) infant T/F Env pseudoviruses were neutralization resistant to paired maternal plasma. Yet, all infant T/F viruses were neutralization sensitive to a panel of HIV-1 broadly neutralizing antibodies and variably sensitive to heterologous plasma neutralizing antibodies. Also, these infant T/F pseudoviruses were overall more neutralization resistant to paired maternal plasma in comparison to pseudoviruses from maternal non-transmitted variants (p = 0.012). Altogether, our findings suggest that autologous neutralization of circulating viruses by maternal plasma antibodies select for neutralization-resistant viruses that initiate peripartum transmission, raising the speculation that enhancement of this response at the end of pregnancy could further reduce infant HIV-1 infection risk.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- UNAIDS. Progress Report on the Global Plan: towards the elimi- nation of new HIV infections among children and keeping their mothers alive UNAIDS, Geneva, Switzerland: 2015.
-
- Dorenbaum A, Cunningham CK, Gelber RD, Culnane M, Mofenson L, Britto P, et al. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA. 2002;288(2):189–98. - PubMed
-
- Wolinsky SM, Wike CM, Korber BT, Hutto C, Parks WP, Rosenblum LL, et al. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992;255(5048):1134–7. - PubMed
-
- Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341(6):394–402. doi: 10.1056/NEJM199908053410602 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

