Effects of Long-Term Denosumab on Bone Histomorphometry and Mineralization in Women With Postmenopausal Osteoporosis
- PMID: 29672714
- PMCID: PMC6037073
- DOI: 10.1210/jc.2017-02669
Effects of Long-Term Denosumab on Bone Histomorphometry and Mineralization in Women With Postmenopausal Osteoporosis
Abstract
Context: Denosumab is a potent antiresorptive agent that reduces fractures in postmenopausal women with osteoporosis.
Objective: Determine effects of up to 10 years of denosumab on bone histology, remodeling, and matrix mineralization characteristics.
Design and setting: International, multicenter, randomized, double-blind trial [Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM)] with a long-term open-label extension.
Patients: Postmenopausal women with osteoporosis (92 women in FREEDOM, 46 in extension) who provided iliac bone biopsies, including 11 who provided biopsies at multiple time points.
Interventions: FREEDOM subjects were randomized 1:1 to subcutaneous denosumab 60 mg or placebo every 6 months for 3 years. Long-term extension subjects continued receiving denosumab, open-label, for 7 additional years.
Outcomes: Bone histology, histomorphometry, matrix mineralization.
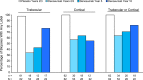
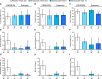
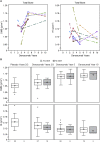
Results: Ten-year denosumab biopsies showed normal histology. Bone histomorphometry indicated normal bone structure and reduced bone remodeling after 10 years of denosumab, similar to levels after 2 and/or 3 and 5 years of denosumab. The degree of mineralization of bone was increased and mineralization heterogeneity was reduced in the denosumab years 2/3 group vs placebo. Changes in these mineralization variables progressed from years 2/3 to year 5 of denosumab, but not thereafter.
Conclusions: Denosumab for 2/3, 5, and 10 years was associated with normal histology, low bone remodeling rate, increased matrix mineralization, and lower mineralization heterogeneity compared with placebo. These variables were unchanged from year 5 to year 10. These data, in combination with the maintenance of low fracture rates for up to 10 years as previously reported with denosumab therapy, suggest that strong, prolonged remodeling inhibition does not impair bone strength.
Trial registration: ClinicalTrials.gov NCT00089791.
Figures


Comment in
-
Letter to the Editor: "Effects of Long-Term Denosumab on Bone Histomorphometry and Mineralization in Women With Postmenopausal Osteoporosis".J Clin Endocrinol Metab. 2018 Jul 1;103(7):2756-2757. doi: 10.1210/jc.2018-00886. J Clin Endocrinol Metab. 2018. PMID: 29771348 No abstract available.
References
-
- Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, San Martin J, Dansey R. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11(5):401–419. - PubMed
-
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C; FREEDOM Trial . Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. - PubMed
-
- Reid IR, Miller PD, Brown JP, Kendler DL, Fahrleitner-Pammer A, Valter I, Maasalu K, Bolognese MA, Woodson G, Bone H, Ding B, Wagman RB, San Martin J, Ominsky MS, Dempster DW; Denosumab Phase 3 Bone Histology Study Group . Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res. 2010;25(10):2256–2265. - PubMed
-
- Kostenuik PJ, Smith SY, Jolette J, Schroeder J, Pyrah I, Ominsky MS. Decreased bone remodeling and porosity are associated with improved bone strength in ovariectomized cynomolgus monkeys treated with denosumab, a fully human RANKL antibody. Bone. 2011;49(2):151–161. - PubMed
-
- Brown JP, Reid IR, Wagman RB, Kendler D, Miller PD, Jensen JE, Bolognese MA, Daizadeh N, Valter I, Zerbini CA, Dempster DW. Effects of up to 5 years of denosumab treatment on bone histology and histomorphometry: the FREEDOM study extension. J Bone Miner Res. 2014;29(9):2051–2056. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

