Glecaprevir/Pibrentasvir Treatment in Liver or Kidney Transplant Patients With Hepatitis C Virus Infection
- PMID: 29672891
- PMCID: PMC6220874
- DOI: 10.1002/hep.30046
Glecaprevir/Pibrentasvir Treatment in Liver or Kidney Transplant Patients With Hepatitis C Virus Infection
Abstract
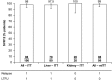
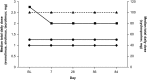
Well-tolerated, ribavirin-free, pangenotypic hepatitis C virus (HCV) treatments for transplant recipients remain a high priority. Once-daily glecaprevir/pibrentasvir demonstrates high rates of sustained virologic response at 12 weeks posttreatment (SVR12) across all major HCV genotypes (GTs). This trial evaluated the safety and efficacy of glecaprevir/pibrentasvir for patients with chronic HCV GT1-6 infection who had received a liver or kidney transplant. MAGELLAN-2 was a phase 3, open-label trial conducted in patients who were ≥3 months posttransplant. Patients without cirrhosis who were HCV treatment-naive (GT1-6) or treatment-experienced (GT1, 2, 4-6; with interferon-based therapy with or without sofosbuvir, or sofosbuvir plus ribavirin) received glecaprevir/pibrentasvir (300/120 mg) once daily for 12 weeks. The primary endpoint compared the percentage of patients receiving glecaprevir/pibrentasvir with SVR12 to a historic SVR12 rate based on the standard of care. Safety of glecaprevir/pibrentasvir was assessed. In total, 80 liver transplant and 20 kidney transplant patients participated in the trial. Most patients had no or minimal fibrosis (80% had fibrosis scores F0-F1) and were infected with HCV GT1 (57%) or GT3 (24%). The overall SVR12 was 98% (n/N = 98/100; 95% confidence interval, 95.3%-100%), which exceeded the prespecified historic standard-of-care SVR12 threshold of 94%. One patient experienced virologic failure. One patient discontinued because of an adverse event considered to be unrelated to treatment; this patient achieved SVR12. Adverse events were mostly mild in severity, and laboratory abnormalities were infrequent.
Conclusion: Once-daily glecaprevir/pibrentasvir for 12 weeks is a well-tolerated and efficacious, ribavirin-free treatment for patients with chronic HCV GT1-6 infection who have received a liver or kidney transplant. (ClinicalTrials.gov NCT02692703.) (Hepatology 2018; 00:000-000).
© 2018 The Authors. Hepatology published by Wiley Periodicals, Inc. on behalf of American Association for the Study of Liver Diseases.
Figures

References
-
- American Association for the Study of Liver Diseases, Infectious Diseases Society of America . Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Published 2017. Accessed November 14, 2017. - PMC - PubMed
-
- Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients . United States organ transplantation. OPTN/SRTR 2012 annual data report. https://srtr.transplant.hrsa.gov/annual_reports/2012/pdf/2012_SRTR_ADR_u.... Published June 2014. Accessed June 14, 2017.
-
- Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, et al. Long‐term outcome of hepatitis C infection after liver transplantation. N Engl J Med 1996;334:815‐820. - PubMed
-
- Garcia‐Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology 2002;35:680‐687. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

