Using disruptive insertional mutagenesis to identify the in situ structure-function landscape of the Shigella translocator protein IpaB
- PMID: 29672980
- PMCID: PMC6153406
- DOI: 10.1002/pro.3428
Using disruptive insertional mutagenesis to identify the in situ structure-function landscape of the Shigella translocator protein IpaB
Abstract
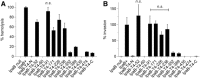
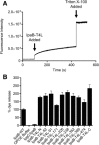
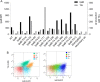
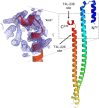
Bacterial type III secretion systems (T3SS) are used to inject proteins into mammalian cells to subvert cellular functions. The Shigella T3SS apparatus (T3SA) is comprised of a basal body, cytoplasmic sorting platform and exposed needle with needle "tip complex" (TC). TC maturation occurs when the translocator protein IpaB is recruited to the needle tip where both IpaD and IpaB control secretion induction. IpaB insertion into the host membrane is the first step of translocon pore formation and secretion induction. We employed disruptive insertional mutagenesis, using bacteriophage T4 lysozyme (T4L), within predicted IpaB loops to show how topological features affect TC functions (secretion control, translocon formation and effector secretion). Insertions within the N-terminal half of IpaB were most likely to result in a loss of steady-state secretion control, however, all but the two that were not recognized by the T3SA retained nearly wild-type hemolysis (translocon formation) and invasiveness levels (effector secretion). In contrast, all but one insertion in the C-terminal half of IpaB maintained secretion control but were impaired for hemolysis and invasion. These nature of the data suggest the latter mutants are defective in a post-secretion event, most likely due to impaired interactions with the second translocator protein IpaC. Intriguingly, only two insertion mutants displayed readily detectable T4L on the bacterial surface. The data create a picture in which the makeup and structure of a functional T3SA TC is highly amenable to physical perturbation, indicating that the tertiary structure of IpaB within the TC is more plastic than previously realized.
Keywords: IpaB; Shigella; translocon; type III secretion.
© 2018 The Protein Society.
Figures


References
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque ASG, Zaidi AKM, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins‐Browne RM, Levine MM (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case‐control study. Lancet 382:209–222. - PubMed
-
- DuPont HL, Levine MM, Hornick RB, Formal SB (1989) Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis 159:1126–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

