Mechanisms of Diabetes-Induced Endothelial Cell Senescence: Role of Arginase 1
- PMID: 29673160
- PMCID: PMC5979610
- DOI: 10.3390/ijms19041215
Mechanisms of Diabetes-Induced Endothelial Cell Senescence: Role of Arginase 1
Abstract
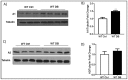
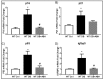
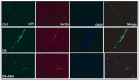
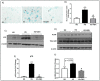
We have recently found that diabetes-induced premature senescence of retinal endothelial cells is accompanied by NOX2-NADPH oxidase-induced increases in the ureohydrolase enzyme arginase 1 (A1). Here, we used genetic strategies to determine the specific involvement of A1 in diabetes-induced endothelial cell senescence. We used A1 knockout mice and wild type mice that were rendered diabetic with streptozotocin and retinal endothelial cells (ECs) exposed to high glucose or transduced with adenovirus to overexpress A1 for these experiments. ABH [2(S)-Amino-6-boronohexanoic acid] was used to inhibit arginase activity. We used Western blotting, immunolabeling, quantitative PCR, and senescence associated β-galactosidase (SA β-Gal) activity to evaluate senescence. Analyses of retinal tissue extracts from diabetic mice showed significant increases in mRNA expression of the senescence-related proteins p16INK4a, p21, and p53 when compared with non-diabetic mice. SA β-Gal activity and p16INK4a immunoreactivity were also increased in retinal vessels from diabetic mice. A1 gene deletion or pharmacological inhibition protected against the induction of premature senescence. A1 overexpression or high glucose treatment increased SA β-Gal activity in cultured ECs. These results demonstrate that A1 is critically involved in diabetes-induced senescence of retinal ECs. Inhibition of arginase activity may therefore be an effective therapeutic strategy to alleviate diabetic retinopathy by preventing premature senescence.
Keywords: arginase; diabetes mellitus; diabetic retinopathy; endothelial cells; hyperglycemia; retina; senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Oubaha M., Miloudi K., Dejda A., Guber V., Mawambo G., Germain M.A., Bourdel G., Popovic N., Rezende F.A., Kaufman R.J., et al. Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci. Transl. Med. 2016;8:362ra144. doi: 10.1126/scitranslmed.aaf9440. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

