The spatio-temporal dynamics of deviance and target detection in the passive and active auditory oddball paradigm: a sLORETA study
- PMID: 29673322
- PMCID: PMC5909247
- DOI: 10.1186/s12868-018-0422-3
The spatio-temporal dynamics of deviance and target detection in the passive and active auditory oddball paradigm: a sLORETA study
Abstract
Background: Numerous studies have investigated the neural underpinnings of passive and active deviance and target detection in the well-known auditory oddball paradigm by means of event-related potentials (ERPs) or functional magnetic resonance imaging (fMRI). The present auditory oddball study investigates the spatio-temporal dynamics of passive versus active deviance and target detection by analyzing amplitude modulations of early and late ERPs while at the same time exploring the neural sources underling this modulation with standardized low-resolution brain electromagnetic tomography (sLORETA) .
Methods: A 64-channel EEG was recorded from twelve healthy right-handed participants while listening to 'standards' and 'deviants' (500 vs. 1000 Hz pure tones) during a passive (block 1) and an active (block 2) listening condition. During passive listening, participants had to simply listen to the tones. During active listening they had to attend and press a key in response to the deviant tones.
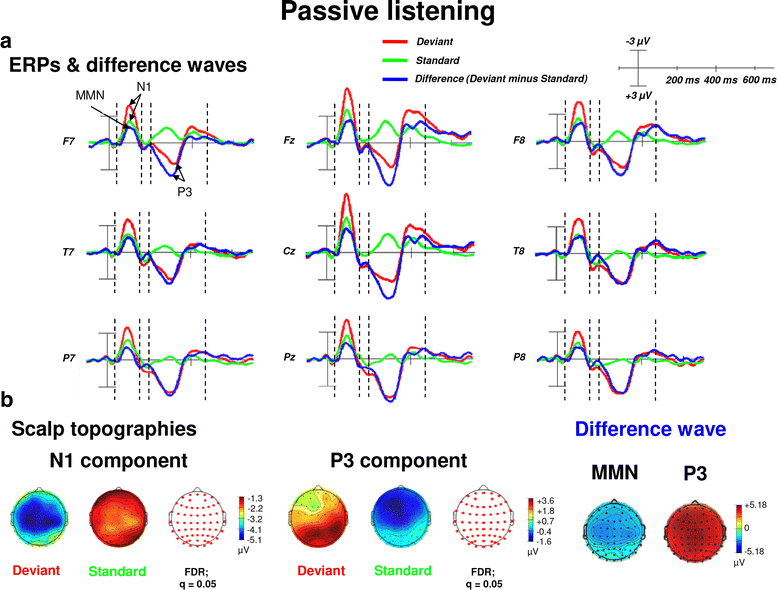
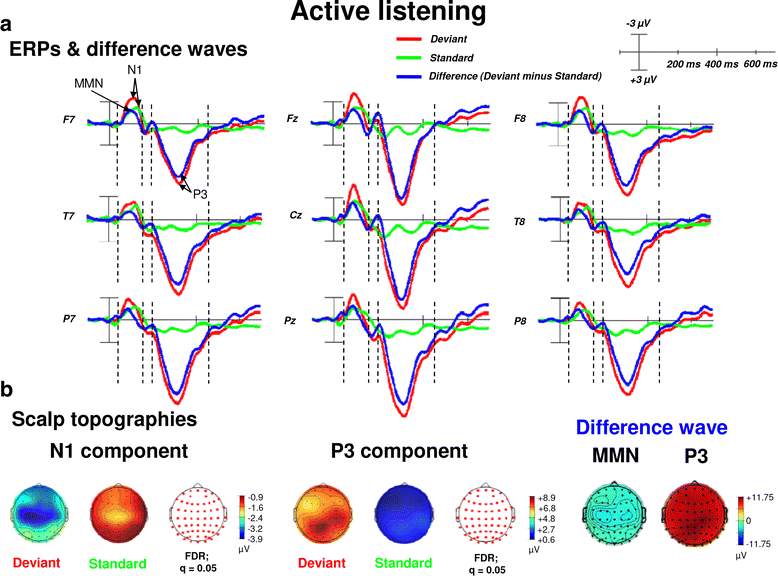
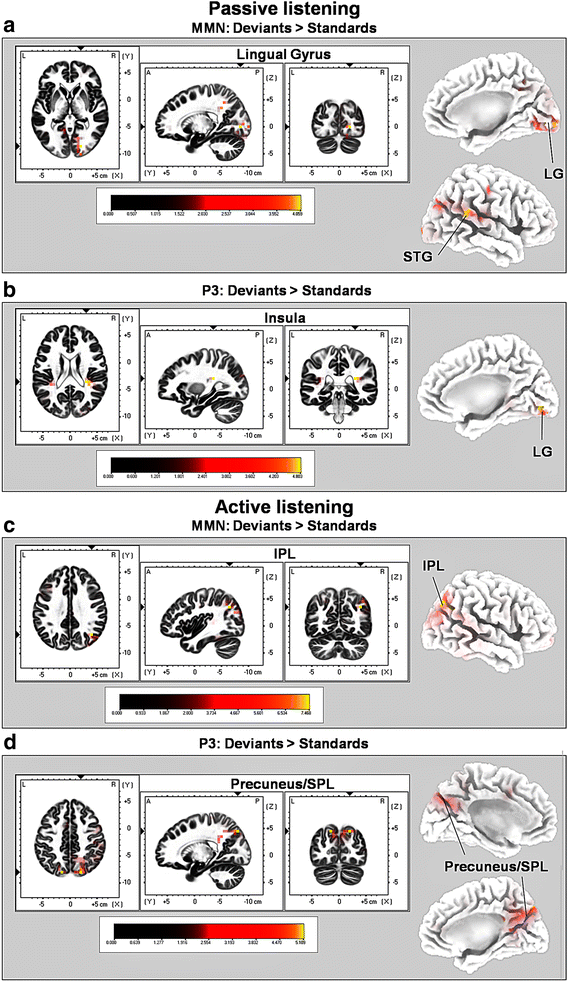
Results: Passive and active listening elicited an N1 component, a mismatch negativity (MMN) as difference potential (whose amplitudes were temporally overlapping with the N1) and a P3 component. N1/MMN and P3 amplitudes were significantly more pronounced for deviants as compared to standards during both listening conditions. Active listening augmented P3 modulation to deviants significantly compared to passive listening, whereas deviance detection as indexed by N1/MMN modulation was unaffected by the task. During passive listening, sLORETA contrasts (deviants > standards) revealed significant activations in the right superior temporal gyrus (STG) and the lingual gyri bilaterally (N1/MMN) as well as in the left and right insulae (P3). During active listening, significant activations were found for the N1/MMN in the right inferior parietal lobule (IPL) and for the P3 in multiple cortical regions (e.g., precuneus).
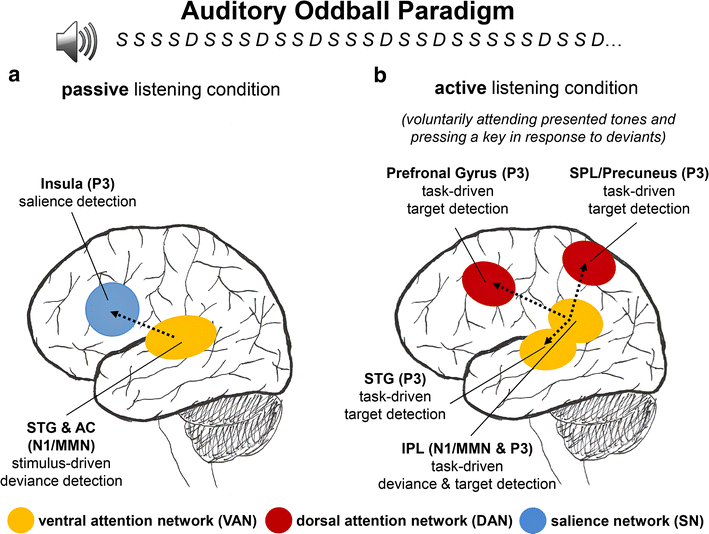
Discussion: The results provide evidence for the hypothesis that passive as well as active deviance and target detection elicit cortical activations in spatially distributed brain regions and neural networks including the ventral attention network (VAN), dorsal attention network (DAN) and salience network (SN). Based on the temporal activation of the neural sources underlying ERP modulations, a neurophysiological model of passive and active deviance and target detection is proposed which can be tested in future studies.
Keywords: Attention; Attention networks; EEG; MMN; Mismatch negativity; N1; P3; Salience; Source localization.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

