Giant Plasma Membrane Vesicles: An Experimental Tool for Probing the Effects of Drugs and Other Conditions on Membrane Domain Stability
- PMID: 29673522
- PMCID: PMC6070695
- DOI: 10.1016/bs.mie.2018.02.007
Giant Plasma Membrane Vesicles: An Experimental Tool for Probing the Effects of Drugs and Other Conditions on Membrane Domain Stability
Abstract
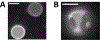
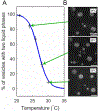
Giant plasma membrane vesicles (GPMVs) are isolated directly from living cells and provide an alternative to vesicles constructed of synthetic or purified lipids as an experimental model system for use in a wide range of assays. GPMVs capture much of the compositional protein and lipid complexity of intact cell plasma membranes, are filled with cytoplasm, and are free from contamination with membranes from internal organelles. GPMVs often exhibit a miscibility transition below the growth temperature of their parent cells. GPMVs labeled with a fluorescent protein or lipid analog appear uniform on the micron-scale when imaged above the miscibility transition temperature, and separate into coexisting liquid domains with differing membrane compositions and physical properties below this temperature. The presence of this miscibility transition in isolated GPMVs suggests that a similar phase-like heterogeneity occurs in intact plasma membranes under growth conditions, albeit on smaller length scales. In this context, GPMVs provide a simple and controlled experimental system to explore how drugs and other environmental conditions alter the composition and stability of phase-like domains in intact cell membranes. This chapter describes methods to generate and isolate GPMVs from adherent mammalian cells and to interrogate their miscibility transition temperatures using fluorescence microscopy.
Keywords: Lipid raft; Liquid-disordered; Liquid-ordered; Miscibility; Phase transition; Plasma membrane; Vesicle.
© 2018 Elsevier Inc. All rights reserved.
Figures
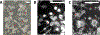



References
-
- Allen JA, Halverson-Tamboli RA and Rasenick MM (2007). “Lipid raft microdomains and neurotransmitter signalling.” Nature Reviews Neuroscience 8(2): 128–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

