Structure and mechanism of mitochondrial electron transport chain
- PMID: 29673555
- PMCID: PMC6138618
- DOI: 10.1016/j.bj.2017.12.001
Structure and mechanism of mitochondrial electron transport chain
Abstract
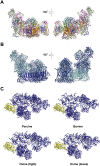
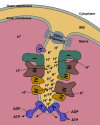
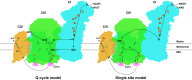
Respiration is one of the most vital and basic features of living organisms. In mammals, respiration is accomplished by respiratory chain complexes located on the mitochondrial inner membrane. In the past century, scientists put tremendous efforts in understanding these complexes, but failed to solve the high resolution structure until recently. In 2016, three research groups reported the structure of respiratory chain supercomplex from different species, and fortunately the structure solved by our group has the highest resolution. In this review, we will compare the recently solved structures of respirasome, probe into the relationship between cristae shape and respiratory chain organization, and discuss the highly disputed issues afterwards. Besides, our group reported the first high resolution structure of respirasome and medium resolution structure of megacomplex from cultured human cells this year. Definitely, these supercomplex structures will provide precious information for conquering the mitochondrial malfunction diseases.
Keywords: Assembly of respirasome; Cristae organization; Electron transfer pathway; Structure of respirasome; Substrate channeling.
Copyright © 2017 Chang Gung University. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. - PubMed
-
- Abrahams J.P., Leslie A.G., Lutter R., Walker J.E. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. - PubMed
-
- Acin-Perez R., Fernandez-Silva P., Peleato M.L., Perez-Martos A., Enriquez J.A. Respiratory active mitochondrial supercomplexes. Mol Cell. 2008;32:529–539. - PubMed
-
- Wu M., Gu J., Guo R., Huang Y., Yang M. Structure of mammalian respiratory supercomplex I1III2IV1. Cell. 2016;167:1598–1609.e10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

