Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms
- PMID: 29673577
- PMCID: PMC6001940
- DOI: 10.1016/j.molmet.2018.03.016
Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms
Abstract
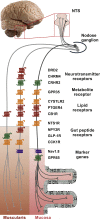
Objectives: G protein-coupled receptors (GPCRs) act as transmembrane molecular sensors of neurotransmitters, hormones, nutrients, and metabolites. Because unmyelinated vagal afferents richly innervate the gastrointestinal mucosa, gut-derived molecules may directly modulate the activity of vagal afferents through GPCRs. However, the types of GPCRs expressed in vagal afferents are largely unknown. Here, we determined the expression profile of all GPCRs expressed in vagal afferents of the mouse, with a special emphasis on those innervating the gastrointestinal tract.
Methods: Using a combination of high-throughput quantitative PCR, RNA sequencing, and in situ hybridization, we systematically quantified GPCRs expressed in vagal unmyelinated Nav1.8-expressing afferents.
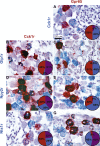
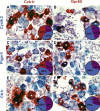
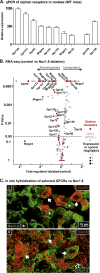
Results: GPCRs for gut hormones that were the most enriched in Nav1.8-expressing vagal unmyelinated afferents included NTSR1, NPY2R, CCK1R, and to a lesser extent, GLP1R, but not GHSR and GIPR. Interestingly, both GLP1R and NPY2R were coexpressed with CCK1R. In contrast, NTSR1 was coexpressed with GPR65, a marker preferentially enriched in intestinal mucosal afferents. Only few microbiome-derived metabolite sensors such as GPR35 and, to a lesser extent, GPR119 and CaSR were identified in the Nav1.8-expressing vagal afferents. GPCRs involved in lipid sensing and inflammation (e.g. CB1R, CYSLTR2, PTGER4), and neurotransmitters signaling (CHRM4, DRD2, CRHR2) were also highly enriched in Nav1.8-expressing neurons. Finally, we identified 21 orphan GPCRs with unknown functions in vagal afferents.
Conclusion: Overall, this study provides a comprehensive description of GPCR-dependent sensing mechanisms in vagal afferents, including novel coexpression patterns, and conceivably coaction of key receptors for gut-derived molecules involved in gut-brain communication.
Keywords: G protein-coupled receptors; GLP1R; Gut hormones; Gut-brain axis; NTSR1; Vagal afferent nerves.
Copyright © 2018 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures




References
-
- Ushiki T. Three-dimensional ultrastructure of the autonomic nerve terminals in the lamina propria mucosae of the rat large intestine. Archives of Histology Cytology. 1992;55(Suppl):87–94. - PubMed
-
- Furness J.B., Rivera L.R., Cho H.J., Bravo D.M., Callaghan B. The gut as a sensory organ. Nature Reviews Gastroenterology & Hepatology. 2013;10(12):729–740. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

