Study protocol: quantitative fibronectin to help decision-making in women with symptoms of preterm labour (QUIDS) part 2, UK Prospective Cohort Study
- PMID: 29674373
- PMCID: PMC5914783
- DOI: 10.1136/bmjopen-2017-020795
Study protocol: quantitative fibronectin to help decision-making in women with symptoms of preterm labour (QUIDS) part 2, UK Prospective Cohort Study
Abstract
Introduction: The aim of the QUIDS study is to develop a decision support tool for the management of women with symptoms and signs of preterm labour, based on a validated prognostic model using quantitative fetal fibronectin (fFN) concentration, in combination with clinical risk factors.
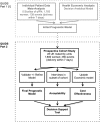
Methods and analysis: The study will evaluate the Rapid fFN 10Q System (Hologic, Marlborough, Massachusetts, USA) which quantifies fFN in a vaginal swab. In QUIDS part 2, we will perform a prospective cohort study in at least eight UK consultant-led maternity units, in women with symptoms of preterm labour at 22+0 to 34+6 weeks gestation to externally validate a prognostic model developed in QUIDS part 1. The effects of quantitative fFN on anxiety will be assessed, and acceptability of the test and prognostic model will be evaluated in a subgroup of women and clinicians (n=30). The sample size is 1600 women (with estimated 96-192 events of preterm delivery within 7 days of testing). Clinicians will be informed of the qualitative fFN result (positive/negative) but be blinded to quantitative fFN result. Research midwives will collect outcome data from the maternal and neonatal clinical records. The final validated prognostic model will be presented as a mobile or web-based application.
Ethics and dissemination: The study is funded by the National Institute of Healthcare Research Health Technology Assessment (HTA 14/32/01). It has been approved by the West of Scotland Research Ethics Committee (16/WS/0068).
Version: Protocol V.2, Date 1 November 2016.
Trial registration number: ISRCTN 41598423andCPMS: 31277.
Keywords: cervix; diagnostic test; fetal fibronectin; pregnancy; preterm birth.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: SJS and JEN work at the University of Edinburgh, who received £1000 sponsorship from Hologic to support a meeting (The Society of Reproductive Investigation and MRC Centre for Reproductive Health Scientific Symposium on Targeting Inflammation to Improve Reproductive Health across the Lifecourse – August 2017). AS has in the past (over last 5 years; not in the last 3 years) received funding for expenses related to advisory board and internal staff education from Hologic. MC received sponsorship from Hologic to organise an educational teaching focusing on prediction of Preterm Birth at the 2017 annual meeting of the British Maternal and Fetal Medicine Society. Hologic, the makers of fFN have provided analysers and technical support for their use to sites participating in the QUIDS prospective cohort study. They have no access to the data, or other involvement in the conduct, data analysis, interpretation of results or decision to publish the results of the study.
Figures
References
-
- Stock SJ, Wotherspoon L, Boyd K, et al. . Study Protocol: Quantitative Fibronectin to help Decision-making in women with Symptoms of Preterm Labour (QUIDS) Part One- Individual Patient Data Meta-analysis and Health Economic Analysis. BMJ Open 2017. doi: 10.1136/bmjopen-2017-020796 [Epub ahead of print]. - DOI - PMC - PubMed
-
- Office for National Statistics (2013). ONS gestation-Specific Infant Mortality in England and Wales 2011. 2017. Cardiff ONS, 2013. Also https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/... (accessed 23 Nov 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical